Table 3.
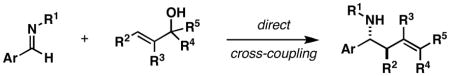 | ||||
|---|---|---|---|---|
| entry | imine | allylic alcohol | yield (%)[a],[b] | product |
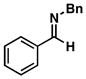
| 1 |
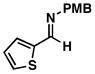 32 |
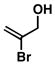 20 |
53 |
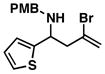 33 |
| 2 |
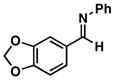 34 |
 35 |
52 |
 36 |
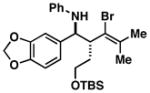
| 3 |
 1 |
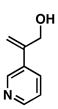 37 |
55 |
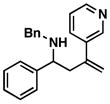 38 |
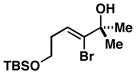
| 4 |
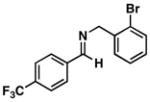 39 |
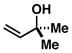 4 |
67 |
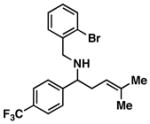 40 |
| 5 |
 41; R1, R2 = Me |
4 | 79 |
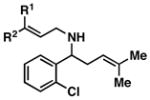 42; R1, R2 = Me |
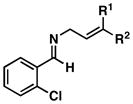
| 6 | 43[c]; R1 = H, R2 = TMS | 4 | 69 | 44; R1 = H, R2 = TMS |
| 7 |
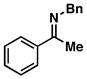 45 |
4 | 83 |
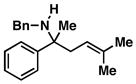 46 |
| 8 | 1 |
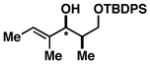 47 |
72 |
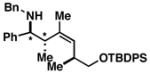 48 dr ≥ 20:1; (Z):(E) ≥ 20:1 |
Reaction conditions: See supporting information for details.
No evidence was found for the production of stereoisomeric products.
Compound 43 was used as a mixture of alkene isomers (E:Z = 4:1).
