Table 2.
Asymmetric synthesis of tertiary benzylic alcohols.a
| entry | product | Mb | yield (%)c |
drd | entry | product | Mb | yield (%)c |
drd |
|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||
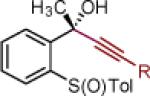
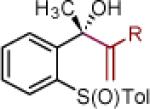
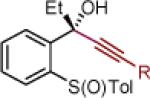
| 1 | 2a: R = Ph | [Ce] | 95 | >50:1 (~50:1) | 15 | 2o: R = CCPh | [Ce] | 78 | >50:1 (~50:1) |
| 2 | 2c: R = 2-CH3-Ph | [Ce] | 88 | >50:1 (~50:1) | 16 | 2p: R = CC-(2-Th) | [Ce] | 82 | >50:1 (33:1) |
| 3 | 2d: R = 6-CH3O-2-Np | [Ce] | 81 | >50:1 (20:1) | 17 | 2q: R = 4-CH3O-Ph | MgBr | 79 | >50:1 (>50:1) |
| 4 | 2e: R = Si(iPr)3 | [Ce] | 87 | >50:1 (>50:1) | 18 | 2r: R = 2-Propenyl | MgBr | 72 | >50:1 (25:1) |
| 5 | 2f: R = CH2OTBS | [Ce] | 65 | 33:1 (12:1) |
|
||||
|
|||||||||
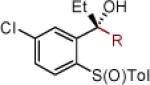
| 6 | 2b: Ar = Ph | MgBr | 83 | >50:1 (~50:1) | 19 | 2s: R = 4-CH3O-Ph | MgBr | 75 | >50:1 (~50:1) |
| 7 | 2g: Ar = 3,5-(CF3)2-Ph | MgBr | 82 | >50:1 (>50:1) | 20 | 2t: R = 6-CH3O-2-Np | MgBr | 79 | >50:1 (>50:1) |
| 8 | 2h: Ar =4-CH3O-Ph | MgBr | 87 | >50:1 (>50:1) | 21 | 2u: R = CCPh | [Ce] | 77 | >50:1 (~50:1) |
|
|
||||||||
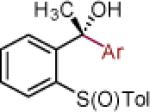
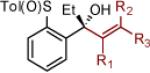
| 9 | 2i: R = H | MgBr | 74 | >50:1 (>50:1) | 22 |
2v: R1 = H R2 = R3 = CH3 |
MgBr | 80) | >50:1 (25:1) |
| 10 | 2j: R = CH3 | MgBr | 90 | >50:1 (33:1) | 23 |
2u: R1 = CH3 R2 = R3 = H |
MgBr | 86 | >50:1 (20:1) |
|
|
||||||||
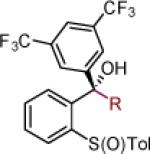
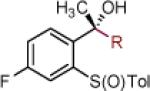
| 11 | 2k: R = Ph | [Ce] | 72 | >50:1 (11:1) | 24 | 2x: R = CCPh | [Ce] | 77 | >50:1 (14:1) |
| 12 | 2l:R = 3,5-(CF3)2-Ph | [Ce] | 81 | >50:1 (14:1) | 25 | 2y: R = Ph | MgBr | 85 | >50:1 (~50:1) |
| 13 | 2m: R = CH2N(CH3)2 | [Ce] | 71 | >50:1 (~50:1) | 26 |
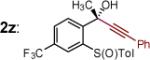
|
[Ce] | 79) | >50:1 (10:1) |
| 14 |
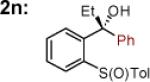
|
MgBr | 84 | >50:1 (>50:1) |
Reaction conditions as in Table 1, entry 4 (M = [Ce]) or 6 (M = MgBr) on a 0.4 mmol scale. The (S)-sulfoxides were used.
[Ce] = reagent derived from alkynyl Li and CeCl3.
Yield of isolated single diastereomer except entry 5 (33:1 dr). The numbers in parenthesis represent conversion of 1 as determined by 1H NMR analysis of crude reaction mixture.
dr determined by 1H NMR analysis of purified material. The numbers in parenthesis represent dr of crude reaction products. TBS = tert-butyldimethyl silyl; Tol = p-tolyl; Np = naphthyl; Th = thienyl.
