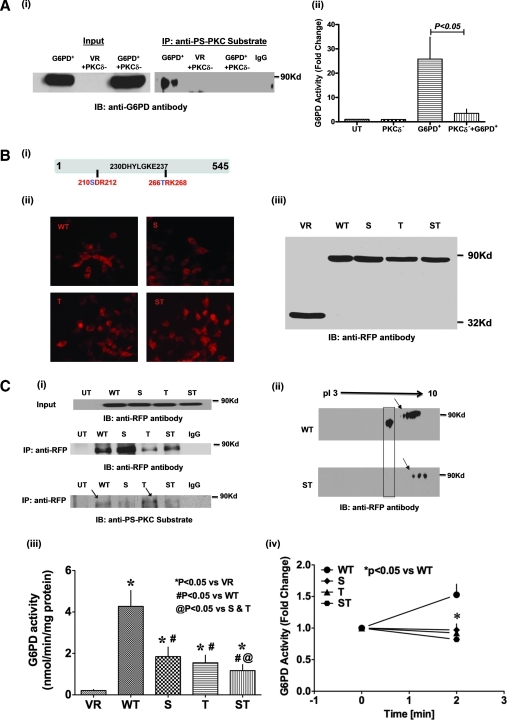
FIG. 4.
Phosphorylation of S210 and T266 residues flanking the active site of G6PD by PKCδ is essential for G6PD activation. (A-i) Coimmunoprecipitation of ectopically overexpressed G6PD-RFP-wt with anti-phosphoserine PKC substrate antibody is abolished by silencing PKCδ (blot is representative of four experiments). Empty vector (VR) is used as a negative control. (A-ii) G6PD activity is significantly greater in HEK 293T17 cells expressing G6PD-RFP-wt than in UT cells (n = 8). Conversely, G6PD activity is significantly suppressed in HEK 293T17 cells transfected with G6PD-RFP-wt+PKCδ siRNA (n = 4–5). (B) Single (S210A, T266A) and double (S210A/T266A) mutations (i) were made within the PKC phosphorylation site flanking the active site of G6PD, and expression of the mutants was confirmed by immunofluorescent microscopy (ii; magnification: 20 × ) and Western blot analysis (iii). (C-i) Western blot done with specific anti-PS-PKC substrate antibody showing in vitro phosphorylation by PKCδ of G6PD-RFP-wt at S210 residue. Note that the anti-PS antibody recognizes phosphorylation of T266A mutant but not the S210A and S210A/T266A mutant. (C-ii) Western blot after 2D electrophoresis shows that G6PD is hypophosphorylated in HEK 293T17 cells expressing the G6PD S210A/T266A double mutant (indicated by an arrow) and that mutating two out of five PKC phosphorylation sites abolished the leftward shift of G6PD (shown in box). (C-iii) Enzymatic activity was suppressed in HEK 293T17 cells expressing either a G6PD single (S210A, T266A) or double (S210A/T266A) mutant (n = 10–12). (C-iv) KCl (30 mM; n = 8)-induced increases in enzymatic activity (at 2 min) were suppressed in HEK 293T17 cells expressing one of the aforementioned G6PD mutants. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).