Abstract
Comprehensive and successful tuberculosis (TB) care and treatment must incorporate effective airborne infection-control strategies. This is particularly and critically important for health care workers and all persons with or at risk of human immunodeficiency virus (HIV) infection. Past and current outbreaks and epidemics of drug-susceptible, multidrug-resistant, and extensively drug-resistant TB have been fueled by HIV infection, with high rates of morbidity and mortality and linked to the absence or limited application of airborne infection-control strategies in both resource-rich and resource-limited settings. Airborne infection-control strategies are available—grouped into administrative, environmental, and personal protection categories—and have been shown to be associated with decreases in nosocomial transmission of TB; their efficacy has not been fully demonstrated, and their implementation is extremely limited, particularly in resource-limited settings. New research and resources are required to fully realize the potential benefits of infection control in the era of TB and HIV epidemics.
Airborne infection-control strategies to prevent Mycobacterium tuberculosis transmission have long been a neglected component of tuberculosis (TB)–control programs, with grave worldwide consequences. Classic guinea pig studies in the 1950s confirmed airborne transmission and quantified exposure risk [1, 2]. Decreasing incidence of TB in high-income countries, coupled with the development of effective anti-TB therapy, reduced risk of transmission of nosocomial TB, with resultant complacency about airborne infection-control measures. Budgetary constraints and the premature belief that TB would be eliminated resulted in the widespread dismantling of TB-control programs.
In the 1980s, coincident with the dramatic increase in the incidence of human immunodeficiency virus (HIV) infection in the United States and elsewhere, an unexpected increase in the incidence of new TB cases occurred as the result of both HIV-induced reactivation of latent TB infection and TB transmission in congregate settings with a high prevalence of HIV infection, such as inner city neighborhoods, drug misuse treatment clinics, and prisons [3]. In addition, dramatic nosocomial outbreaks of multidrug-resistant (MDR) TB (defined as resistance to at least the first-line isoniazid and rifampin) occurred in industrialized countries and were closely associated with the progressing HIV and AIDS epidemic. These HIV infection–associated TB outbreaks were characterized by spread to both patients and health care workers (HCWs) and extremely high mortality [4, 5]. They required massive emergency infusions of resources to turn the tide and regain TB control [6].
The prevalence of both drug-susceptible and drug-resistant TB appeared to decrease again in industrialized countries and recede from public and professional interest as highly active antiretroviral therapy (HAART) became available and TB programs strengthened airborne infection control. In many resource-limited settings, however, particularly in Africa, Asia, and the countries of the former Soviet Union, rates of drug-susceptible and drug-resistant TB continued to increase, and TB reemerged as a serious global public health threat. World Health Organization (WHO) surveillance revealed high and increasing rates of drug resistance in retreatment cases and, alarmingly, also in new, previously untreated TB cases [7]. More than half of the estimated 500,000 global MDR-TB cases diagnosed in 2007 were believed to have resulted from primary transmission of drug-resistant organisms [8].
It was the dramatic occurrence of 2 seemingly unrelated events, however, that finally brought the issue of transmission of drug-resistant TB, its relationship to the HIV epidemic, and the global absence of adequate TB infection control into glaring public, medical, and scientific view. First, in an uncanny and tragic historic replay of the previous MDR-TB outbreaks, the new report of extensively drug-resistant (XDR) TB (defined as MDR-TB plus resistance to any fluoroquinolone and any of the injectable second-line agents) in rural KwaZulu Natal Province, South Africa, called attention to the dangers of nosocomial TB transmission in the presence of HIV infection and AIDS and the consequences of limited or no attention to infection control. XDR-TB was reported in 53 patients from a setting with a high prevalence of TB and HIV infection and a hospital with large congregate TB wards similar to those in most resource-limited settings [9]. Reminiscent of the earlier outbreaks, all patients tested were HIV infected, and there was almost complete and rapid mortality. Nosocomial transmission was apparent because most patients had not previously been treated for TB and two-thirds had been hospitalized in the preceding 2 years. In addition, 85% of tested XDR Mycobacterium tuberculosis isolates were of a similar genetic family, and several HCWs were among the persons who were infected and died. Additional studies provided more support for nosocomial transmission, including the demonstration of exogenous XDR M. tuberculosis superinfection in patients with TB and HIV coinfection [10], clustered genotypes in MDR and XDR M. tuberculosis isolates from HIV-infected patients [11], and increasing recognition of the presence of MDR-TB and XDR-TB throughout South Africa and neighboring countries. In these settings, poor or absent airborne infection control and high prevalence of HIV infection has facilitated a nosocomially transmitted epidemic of unprecedented proportions [12].
The second event involved an HIV-uninfected individual with MDR-TB who traveled on multiple transatlantic and European flights. The episode received extensive media coverage and raised awareness of drug-resistant TB in developed countries and the possibility of airborne transmission not only in health care facilities but also in a broader array of public settings. In addition to the fear of transmission of untreatable TB, these 2 events vividly revealed the longstanding collective neglect and deficiencies in TB prevention, including infection control, diagnosis, and treatment, and the great need for resources and renewal of global interest in TB control.
The MDR-TB and XDR-TB epidemic has raised concerns about the possibility of TB disease for which there are limited or no treatment options. Even in the United States and other developed countries, the lack of treatment options could endanger already diminished resources for TB control and potentially result in another disastrous surge of both drug-resistant and drug-susceptible TB. In New York City alone, reversing the 1980s TB epidemic cost >$1 billion [6]. Because of the intertwined relationship with HIV infection, however, the greatest threat is in resource-limited countries with a high prevalence of TB and HIV infection. This risk is compounded by the pooling of patients with HIV infection and TB in congregate health care settings. Although widespread expansion of HIV services and integration of HIV infection and TB care and treatment is of unquestioned benefit, an increased opportunity for transmission of both drug-susceptible and drug-resistant TB is inevitable without proper attention to infection control. In settings with a high burden of TB, up to 10% of HIV-infected persons may have previously undiagnosed active TB at the time of HIV testing [13], and recent HIV clinic surveys have shown astounding rates of undiagnosed active TB, including drug-resistant TB [14]. In resource-limited settings, the magnitude of the TB and HIV epidemics is inversely proportional to the availability of resources. As a consequence, in addition to enormous individual morbidity and mortality, the success of both TB-control programs and the historic growth of HIV/AIDS treatment programs are threatened [10, 15].
THE SPECIAL ISSUE OF HCWS
HCWs are at particularly high risk of M. tuberculosis infection and TB disease. Nosocomial TB transmission to HCWs further deepens the already severe human resources crisis in global health and in HIV and TB services. In a review of M. tuberculosis infection and TB disease in HCWs, the median annual incidence of occupationally acquired TB was 5.8% (range, 0%–11%) and 1.1% (range, 0.2%–12%) in low- and high-income countries, respectively; was consistently higher than TB incidence in the general population; and was linked to degree of TB exposure and the presence or absence of airborne infection control [16, 17].
STRATEGIES FOR AIRBORNE INFECTION CONTROL
Poor or absent infection-control programs in health care facilities is unacceptable and avoidable [18]. The Centers for Disease Control and Prevention and WHO have formulated TB infection-control guidelines for both resource-rich and resource-limited nations on the basis of a 3-tiered approach of controls: (1) administrative or work practice, (2) environmental, and (3) personal protection [19–22]. These guidelines are intended to work synergistically at both the public health policy and local facility implementation levels.
Administrative controls
Infection-control administrative measures should be instituted at several health care system levels (Table 1). It is imperative that infection control be a visible and integral part of the national TB and HIV programs. This includes resources and dedicated trained staff for infection-control activities in all health care facilities. National TB infection-control responsibilities should include assessment of all new health care facility construction to ensure that design and building are performed in accordance with infection-control standards for prevention of nosocomial TB transmission. For example, design that optimizes patient flow and natural ventilation is best incorporated early in the planning stage. However, feasible and practical engineering measures can still be accomplished through modification of existing infrastructure, although this is often prohibitively expensive in many countries. Furthermore, the national program should develop and maintain a strict monitoring and evaluation system, engage in advocacy efforts regarding infection control, and coordinate and conduct operational research regarding infection control efficiency and cost-effectiveness. Finally, in the era of HIV/AIDS, it is essential that these activities are harmonized with policies and practice directed at the safety and protection of staff and patients with TB and HIV coinfection.
Table 1.
Policy Needs for Control of Tuberculosis (TB) Transmission in the Context of HIV and Antiretroviral Therapy Rollouts
| Policy need |
|---|
| Recognize necessity for strong and effective airborne infection control as part of comprehensive TB and HIV policy, programs, and practices at country-, facility-, and community-specific levels |
| Provide resources for comprehensive airborne infection-control programs to protect patients and health care workers |
| Require incorporation of development, implementation, and monitoring of infection control in funding for TB and HIV programs supported by international and national donors and funding agencies (eg, Global Fund to Fight AIDS, Tuberculosis and Malaria and US President’s Emergency Plan for AIDS Relief) |
| Develop guidance for community-level TB infection-control measures, especially in congregate settings |
| Provide firm public health policies to ensure that patients receive treatment and comprehensive clinical TB and HIV care and decrease risk to the public while preserving the rights of individual patients and health care workers |
| Provide flexible and setting-specific treatment options, including community-based therapy, to allow patients to receive therapy for TB, multidrug-resistant TB, and extensively drug-resistant TB as outpatients |
| Support TB infection-control research |
At the health care facility level, administrative control measures are the first line of defense against TB transmission and are intentioned to implement and monitor strategies to reduce generation of infectious particles to decrease staff and patient TB exposure [13, 21]. This requires development of a facility-specific infection-control policy modeled on the national TB program plan, with modifications appropriate to the local setting [22]. Each facility should designate dedicated staff, with the support and authority at the facility to accomplish these tasks. Essential program components include the prompt recognition, separation, provision of services, and referral of persons with potentially infectious TB. Specific administrative controls vary according to setting. In resource-rich settings, persons suspected of having TB and patients who have received a diagnosis of TB are usually placed in individual isolation; in low-resource settings, relocation to well-ventilated areas and application of cough hygiene, decreasing the duration of hospitalization, and greater provision of outpatient TB treatment may effectively reduce the potential for nosocomial transmission.
Environmental controls
Several strategies are available to reduce exposure to infectious particles, including natural ventilation, mechanical ventilation, and upper-room ultraviolet (UV) light. After many years of relative disinterest in these strategies, they are now increasingly informed by recent and important studies on airborne TB transmission among both HIV-infected and HIV-uninfected patients [23–27]. Mechanical ventilation delivering negative pressure and 12 air changes/h is the standard of care for respiratory TB isolation [21], but such systems need careful design and are expensive to install. More importantly, they require ongoing maintenance, necessitating both resources and expertise. Poorly maintained mechanical ventilation systems have been widely documented in resource-rich settings [28, 29] and implicated in several TB outbreaks [30–32]. It is important that the budget for any new installation includes adequate provision for ongoing maintenance costs. Furthermore, high air-exchange mechanical ventilation is limited by cost to certain settings deemed high risk for TB transmission, such as respiratory isolation rooms. However, congregate settings, such as HIV programs, waiting rooms, outpatient departments, and emergency departments, are where patients with undiagnosed, untreated, and, most likely, infectious TB are often found. TB infection control in these frequently overcrowded settings is paramount, and alternative environmental control measures are required.
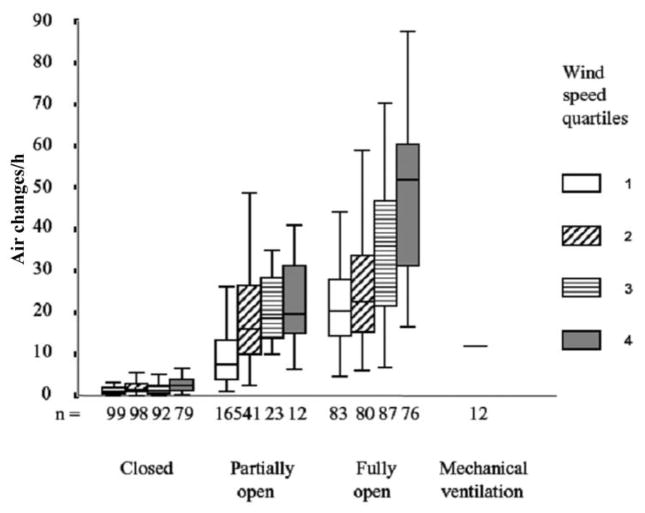
Natural ventilation, which informed the design of TB sanatoria with high ceilings and large windows in an era when fresh air therapy was treatment for TB, currently offers an attractive tool in combating TB transmission in health care settings. A 2007 Peruvian study investigated rates of fresh air exchange achievable by natural means in health care settings [25]. More than 70 clinical rooms where patients with TB may be found were studied, including emergency departments and outpatient clinics (Figure 1). Opening windows and doors provided a median of 28 air changes/h, increasing to 40 air changes/h when only older facilities with high ceilings and large windows were evaluated. Determinants of ventilation included wind speed, window size, and cross-ventilation. Although TB transmission was not directly measured, likely reductions in risk of TB transmission were shown through use of validated TB models. The mechanically ventilated TB-HIV unit evaluated in this study delivered <3 air changes/h, despite having been established <2 years previously. A recent Thai study had similar findings, with high rates of air exchange recorded in naturally ventilated facilities and most mechanical ventilation decreasing below standards [33]. Finally, in the district hospital in KwaZulu Natal, where the XDR-TB epidemic was first detected, subsequent studies indicated that, when all windows were closed in the TB ward, <1 air change/h occurred; newly installed mechanical ventilation could provide up to 15 air changes/h, but opening all windows and doors and using mixing fans produced >60 air changes/h (T Moll, personal communication).
Figure 1.
The effect of opening increasing numbers of windows and doors and of wind speed on rates of natural ventilation in health care facilities. Natural ventilation was measured in 368 experiments in 70 rooms in which patients with tuberculosis (TB) might be found in 8 hospitals in Lima, Peru. These included respiratory isolation rooms (n =13); wards for TB (n =13), respiratory (n =9), general medical (n =8), and HIV and infectious diseases (n =4) patients; emergency departments (n =8); outpatient consulting rooms (n =6); TB clinics (n =5); nebulizer rooms (n =2); an autopsy room; and a respiratory outpatient waiting room. Five hospitals were built before 1950, and 3 were constructed during 1970–1990. Wind speed was measured at the window; “n” refers to the number of experiments.
Natural ventilation has several advantages, including relatively low cost, low maintenance, and applicability to a wide variety of settings, including large areas, such as waiting rooms and outpatient departments. However, it is climate dependent, and windows may often be tightly closed during winter months or at night. Indeed, a median of 1.5 air changes/h was measured in the Peru study with windows and doors closed, with correspondingly high calculated risk of TB transmission [25]. Careful use of skylights or permanently open small windows, however, may provide sufficient baseline ventilation without undue discomfort for room occupants. Natural ventilation is likely to be most effective for airborne infection control when it is included at the architectural design stage of health care facility planning.
M. tuberculosis is susceptible to UV light, and the use of shielded fixtures that flood the upper room with high intensity UV light without endangering occupants in the lower room has long been advocated for TB infection control. With adequate room air mixing provided by convection currents, fans, or ventilation systems, infectious droplet nuclei produced by coughing patients are likely to pass through the UV field and be sterilized. The efficacy of upper-room UV light has been demonstrated in various studies using aerosol chambers and mock isolation rooms; however, despite being recommended in guidelines, use is not widespread owing in part to a lack of clinical data [34–36]. In a recent Peruvian study [26], upper-room UV lights installed in a TB-HIV ward, in addition to small fans to assist air mixing, reduced airborne TB transmission by 70%. These findings have been corroborated by similar studies in South Africa, which demonstrated ~80% efficacy of upper-room UV light (E Nardell, personal communication). Upper-room UV light requires expert design and UV field checking after installation, but maintenance is relatively simple, and it is generally a safe intervention [26, 37] Upper-room UV light offers several advantages for TB infection control; it is climate independent, relatively low cost, and applicable to large areas, such as waiting rooms.
Personal controls
An essential personal protective practice is regular and proper wearing of N95 respirator masks. Although there is limited direct evidence of effectiveness [20], respirators are the best currently available method of guarding against inhalation of TB bacilli. Effective protection requires fit testing to ensure the correct size and habitual, routine use in environments with risk of TB (usually TB wards, HIV clinics, directly observed short-course treatment offices, and others), as designated by the facility-specific plan. For most resource-limited settings, ensuring a constant supply is essential, but the main challenge lies in ensuring regular compliance by staff.
HIV testing should be offered and encouraged for all HCWs. Stigma, lack of confidentiality, fear of job and income loss, and lack of treatment options have historically discouraged HCWs from knowing their HIV status [13, 38]. Additional staff protective measures include mandatory screening for active TB disease at the start of employment and at regular intervals, education and encouragement to seek evaluation for symptoms of TB, provision of personal respirators, and paid sick leave as needed. HCWs found to be HIV infected should be provided access to HIV care and HAART and, for those who are working in an area with high TB risk, offered discreet transfer to a low-risk area of the health care facility.
Many infection-control practices are dependent on HCW implementation and usually imply modifying and sustaining behavioral changes. Infection-control practice would benefit greatly from more collaborative and sophisticated input from the behavioral sciences [39].
EXTENSION OF INFECTION CONTROL TO NON–HEALTH CARE SETTINGS
The aforementioned recommendations and guidelines focus largely on reduction of risk of TB transmission in health care facilities. The Centers for Disease Control and Prevention and the WHO have now recognized the importance of broadening the environments in which TB transmission might occur and for which airborne infection control is essential. Therefore, the appellation “settings” has replaced “facilities” and includes HIV testing centers, drug treatment programs, correctional institutions, and other community congregate settings [20, 22]. Mathematical modeling of the XDR-TB epidemic in rural South Africa [40] concluded that the epidemic has likely spread to community settings, and therefore, community-based infection-control strategies in parallel to those in hospital facilities are required. Household contact tracing has been shown to improve TB case detection, but most resource-limited settings have insufficient resources to perform comprehensive and sustained household contact studies or focus on household contact infection control. Formal guidelines in other congregate community settings, such as public transportation, taxi ranks, bars, and social gatherings, are lacking and needed.
EVIDENCE FOR EFFECTIVENESS OF INFECTION-CONTROL STRATEGIES
Numerous studies have shown that implementation of recommended TB infection-control strategies has been associated with reduced outbreaks of TB in health care facilities [17]. Evaluation of the efficacy of infection-control interventions, however, has been challenging. Effectiveness assessments often focus on process measures; however, reduction in transmission is more difficult to directly attribute causally to infection-control strategies, although studies using guinea pigs to measure the infectiousness of air have been helpful. Furthermore, because multiple strategies are performed simultaneously, the effectiveness of individual infection-control measures in reducing TB transmission is difficult to determine. Finally, in contrast to high-income countries, little information is available about implementation and effectiveness of airborne infection-control measures in resource-limited settings [41, 42].
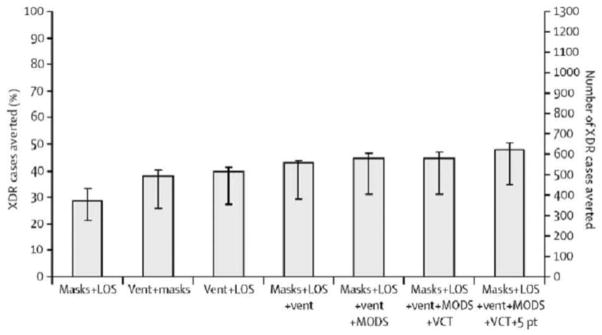
Because of the urgency of reducing the risk of drug-susceptible and drug-resistant TB transmission both to patients and staff in health care settings with a high prevalence of TB and HIV infection, documentation of the efficacy and cost-effectiveness of these measures is of critical importance (Table 2). Use of mathematical modeling with existing data to estimate the potential benefit of infection-control strategies in a resource-poor setting with a high prevalence of TB and HIV infection has shown that combinations of inexpensive and readily available administrative, environmental, and personal protection strategies could avert a substantial proportion of the anticipated 1300 new XDR-TB cases in the next 5 years (Figure 2) [43]. A combination of rapid diagnostics, decreased hospitalization, improved natural ventilation, HIV testing and HAART initiation, cohorting in 5 patient clusters, and respirator use averted an estimated 625 (48%) of the 1300 anticipated cases. In addition, these strategies averted an estimated 75% of anticipated HCW cases. Preliminary subsequent observational data at the same site have shown the feasibility of implementation of administrative, environmental, and personal controls; monitoring of process measures; and a reduction in the prevalence of MDR-TB and XDR-TB in the inpatient hospital wards over a 4-year period [44].
Table 2.
Research Priorities for Tuberculosis (TB) Infection Control in the Context of HIV and Antiretroviral Therapy Rollouts
| Research needs |
|---|
| Support basic and applied research to investigate airborne TB transmission dynamics and strategies to reduce individual patient infectiousness |
| Support operational research to (1) develop and validate process and outcome indicators for airborne infection control, (2) investigate the efficacy and cost-effectiveness of infection-control strategies, and (3) investigate household and community-level interventions for TB infection control |
| Engineering research to develop air handling and facility improvements, including optimizing natural ventilation in health care settings, for cold or hot climates, low cost ultraviolet light fixtures for widespread use in resource-limited settings, and potential for mixed-mode ventilation systems |
| Behavioral research to develop effective strategies to inform, motivate, and provide skills to health care workers to implement and sustain effective airborne infection-control procedures and practices |
Figure 2.
Extensively drug-resistant (XDR) tuberculosis (TB) cases averted during 2007–2012 in Tugela Ferry, South Africa, with use of combinations of available strategies [43]. Efficacy of rapidly available combinations of strategies to reduce nosocomial transmission of XDR-TB. 5 pt, isolation of patients in groups of 5 patients; LOS, reduction in mean duration of stay to 5 days; Mask, both staff N95 respirators and patient masks with adherence enforcement; MODS, microscopic observed drug-susceptibility assay; VCT, voluntary counseling and testing of admitted patients, with subsequent antiretroviral therapy to those who qualify; Vent, improvements in natural ventilation.
CONCLUSIONS
Prevention and interruption of airborne transmission of drug-susceptible and drug-resistant TB in the era of HIV infection and HAART rollouts requires comprehensive airborne infection-control strategies. Such strategies have been neglected, but guidelines have been developed for resource-rich and resource-limited settings. Resources are needed for their implementation and for research to demonstrate both efficacy and effectiveness.
Acknowledgments
Financial support. Fogarty International Center, National Institutes of Health (R24TW007988, the Fogarty International Clinical Research Scholars Support Center), Gilead Foundation, The Doris Duke Charitable Foundation, The Irene Diamond Fund, the President’s Emergency Program for AIDS Relief, and the Wellcome Trust.
Supplement sponsorship. This article is part of a supplement entitled “Synergistic Pandemics: Confronting the Global HIV and Tuberculosis Epidemics,” which was sponsored by the Center for Global Health Policy, a project of the Infectious Diseases Society of America and the HIV Medicine Association, through a grant from the Bill & Melinda Gates Foundation.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Riley R. Aerial dissemination of pulmonary tuberculosis. Am Rev Tuberc. 1957;76:931–941. doi: 10.1164/artpd.1957.76.6.931. [DOI] [PubMed] [Google Scholar]
- 2.Riley R, Mills C, O’Grady F, Sultan L, Wittstadt F, Shivpuri D. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 3.Alland D, Kalkut G, Moss A, et al. Transmission of tuberculosis in New York City—an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 4.Frieden T, Sterling T, Pablos-Mendez A, et al. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 5.Edlin B, Tokars J, Grieco M, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 6.Frieden T, Fujiwara P, Washko R, Hamburg M. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333:229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Anti-tuberculosis drug resistance in the world: third global report. Geneva: World Health Organization; 2004. [Google Scholar]
- 8.World Health Organization. Antituberculosis drug resistance in the world: fourth global report. Geneva: World Health Organization; 2008. [Google Scholar]
- 9.Gandhi N, Moll A, Sturm A, et al. Extensively drug-resistant tuberculosis (XDR TB) as a cause of death among TB/HIV co-infected patients in a rural area in South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 10.Andrews J, Gandhi N, Moodley M, et al. Exogenous re-infection with multidrug- and extensively drug-resistant tuberculosis among patients receiving treatment for tuberculosis in rural KwaZulu Natal, South Africa. J Infect Dis. 2008;198:1582–1589. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi N, Shenoi S, Weissman D, et al. Low genetic diversity among MDR & XDR TB: isolates from Tugela Ferry, South Africa, 2005–2006. Program and abstracts of the American Thoracic Society Annual Conference; San Diego. 2009. [Google Scholar]
- 12.Andrews J, Shah N, Gandhi N, Moll A, Friedland G. Multidrug-resistant and extensively drug-resistant tuberculosis: implications for the HIV epidemic and antiretroviral therapy roll-out in South Africa. J Infect Dis. 2007;196:S482–S490. doi: 10.1086/521121. [DOI] [PubMed] [Google Scholar]
- 13.Bock N, Jensen P, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis. 2007;196:S108–S113. doi: 10.1086/518661. [DOI] [PubMed] [Google Scholar]
- 14.Babaria P, Shah N, Moll A, Gandhi N, Friedland G. High rate of unrecognized tuberculosis and drug-resistant tuberculosis among antiretroviral clinic patients in rural South Africa. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. Abstract 782. [Google Scholar]
- 15.Shenoi S, Heysell S, Moll T, Friedland G. Multidrug resistant and extensively drug resistant TB: consequences for the global HIV community. Curr Opin Infect Dis. 2009;22:11–17. doi: 10.1097/QCO.0b013e3283210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi R, Reingold A, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med. 2006;3:e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007;11:593–605. [PubMed] [Google Scholar]
- 18.Friedland G. Protecting health care workers; the critical role of airborne infection control. Int J Tuberc Lung Dis. 2008;12:585. [PubMed] [Google Scholar]
- 19.World Health Organization. Tuberculosis infection control in the era of expanding HIV care and treatment: an addendum to WHO guidelines for the prevention of tuberculosis in heath care facilities in resource limited settings, 1999. Atlanta: World Health Organization, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 20.World Health Organization. Policy on TB infection control in health-care facilities, congregate settings and households. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Jensen P, Lambert L, Iademarco M, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Morb Mortal Wkly Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Plan to combat extensively drug-resistant tuberculosis recommendations of the Federal Tuberculosis Task Force. MMWR Morb Mortal Wkly Rep. 2009:58. [PubMed] [Google Scholar]
- 23.Escombe A, Oeser C, Gilman R, et al. The detection of airborne TB transmission from HIV infected patients using an in vivo air sampling model. Clin Infect Dis. 2007;44:1349–1357. doi: 10.1086/515397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escombe AR, Moore DA, Gilman RH, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5(9):e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4(2):e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escombe A, Moore D, Gilman R, et al. Upper-room ultraviolet light and negative air ionization to prevent TB transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nardell E, Bucher S, Brickner P, et al. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 2008;123:52–60. doi: 10.1177/003335490812300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavelchak N, DePersis R, London M, et al. Identification of factors that disrupt negative air pressurization of respiratory isolation rooms. Infect Control Hosp Epidemiol. 2000;21:191–195. doi: 10.1086/501742. [DOI] [PubMed] [Google Scholar]
- 29.Fraser V, Johnson K, Primack J, Jones M, Medoff G, Dunagan W. Evaluation of rooms with negative pressure ventilation used for respiratory isolation in seven midwestern hospitals. Infect Control Hosp Epidemiol. 1993;14:623–628. doi: 10.1086/646654. [DOI] [PubMed] [Google Scholar]
- 30.Pearson M, Jereb J, Frieden T, et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis: a risk to patients and health care workers. Ann Intern Med. 1992;117:191–196. doi: 10.7326/0003-4819-117-3-191. [DOI] [PubMed] [Google Scholar]
- 31.Menzies D, Fanning A, Yuan L, FitzGerald J the Canadian Collaborative Group in Nosocomial Transmission of TB. Hospital ventilation and risk for tuberculosis infection in canadian health care workers. Ann Intern Med. 2000;133:779–789. doi: 10.7326/0003-4819-133-10-200011210-00010. [DOI] [PubMed] [Google Scholar]
- 32.Beck-Sague C, Dooley S, Hutton O, et al. Hospital outbreak of multidrug resistant Mycobacterium tuberculosis infections: factors in transmission to staff and HIV-infected patients. JAMA. 1992;268:1280–1286. doi: 10.1001/jama.1992.03490100078031. [DOI] [PubMed] [Google Scholar]
- 33.Jiamjarasrangsi W, Bualert S, Chongthaleong A, Chaindamporn S, Udomsantisuk N, Euasamarnjit W. Inadequate ventilation for nosocomial tuberculosis prevention in public hospitals in Central Thailand. Int J Tuberc Lung Dis. 2009;13:454–459. [PubMed] [Google Scholar]
- 34.Jensen P. Where should infection control programs for tuberculosis begin? Int J Tuberc Lung Dis. 2005;9:825. [PubMed] [Google Scholar]
- 35.Stead W, Yeung C, Hartnett C. Probable role of ultraviolet irradiation in preventing transmission of tuberculosis: a case study. Infect Control Hosp Epidemiol. 1996;17:11–13. doi: 10.1086/647182. [DOI] [PubMed] [Google Scholar]
- 36.Iseman M. A leap of faith: what can we do to curtail intrainstitutional transmission of tuberculosis? Ann Intern Med. 1992;117:251–253. doi: 10.7326/0003-4819-117-3-251. [DOI] [PubMed] [Google Scholar]
- 37.First M, Weker R, Yasui S, Nardell E. Monitoring human exposures to upper-room germicidal ultraviolet irradiation. J Occup Environ Hyg. 2005;2:285–292. doi: 10.1080/15459620590952224. [DOI] [PubMed] [Google Scholar]
- 38.Kanjee Z, Catterick K, Moll A, Shah S, Gandhi N, Friedland G. Multi-and extensively-drug resistant tuberculosis infection control assessment in a resource-limited setting in rural South Africa. Program and abstracts of the XVII International AIDS Conference; Mexico City. 2008. Abstract CDB0104. [Google Scholar]
- 39.Pittet D. The Lowbury lecture: behaviour in infection control. J Hosp Infect. 2004;58:1–13. doi: 10.1016/j.jhin.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Basu S, Friedland G, Medlock J, et al. Averting epidemics of extensively drug-resistant tuberculosis. Proc Natl Acad Sci U S A. 2009;106:7672–7677. doi: 10.1073/pnas.0812472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harries A. Practical and affordable measures for the protection of healthcare workers from tuberculosis in low-income countries. Bull World Health Organ. 1997;75:477–489. [PMC free article] [PubMed] [Google Scholar]
- 42.Harries A, Hargreaves N, Gausi F, Kwanjana J, Salaniponi F. Preventing tuberculosis among health workers in Malawi. Bull World Health Organ. 2002;80:526–531. [PMC free article] [PubMed] [Google Scholar]
- 43.Basu S, Andrews J, Poolman E, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catterick K, Shenoi S, Moll A, et al. Feasible and effective control program to limit nosocomial transmission of multiple and extensively drug resistant (MDR and XDR) TB in Tugela Ferry. Program and abstracts of the South African AIDS Conference; Durban, South Africa. 2009. [Google Scholar]