Abstract
Pacemaker endocarditis remains a rare but potentially life threatening complication of pacemaker implantation. This case illustrates a rare cause of pacemaker endocarditis, Serratia marcescens, the management difficulties that can be faced with such organisms, and the potential indolent nature of pacemaker lead associated endocarditis. A review of the current data for pacemaker endocarditis management suggests that treatment with antimicrobials alone is unlikely to be curative and explantation of the device is recommended in all cases of confirmed pacemaker endocarditis (by echocardiography, in correlation with the patient’s clinical condition and inflammatory markers).
Background
Pacemaker endocarditis remains a rare but potentially life threatening complication of pacemaker implantation. The principles of managing patients with this specific cause of infection is generally poorly understood, despite pacemakers and implantable cardiac defibrillators becoming more commonplace in modern practice within cardiology. Highlighting the basic principles that underpin management of this particular type of endocarditis was the drive to report on this particular case. Furthermore, the case demonstrates the variety of pathological organisms that are implicated in pacemaker endocarditis and their possible indolent nature, and the subsequent difficulty in diagnosis and treatment, highlighting the importance of management being guided by a multifaceted team including cardiologists, microbiologists and cardiothoracic surgeons.
Case presentation
A 67-year-old man presented with a 12 month history of recurrent episodes of fever, malaise and fatigue. He had been admitted three times with these symptoms to his local hospital. On each occasion no focal source of infection was identified despite a routine septic screen (including urine, blood cultures and chest radiography). The fever was treated empirically with broad spectrum antibiotics (cephalosporins and aminoglycosides), to which the patient responded well on each occasion. A total of seven peripheral blood cultures were taken during these admissions, often after initiation of antibiotic treatment. An initial screening transthoracic echocardiogram (TTE) reported no visible vegetations. No invasive procedures were required during the initial presentations (for example, urinary catheters, central venous lines). His medical background included pulmonary sarcoidosis diagnosed 25 years before, which was now quiescent. He had a dual chamber pacemaker implanted in 1993 for symptomatic sinus bradycardia. A routine box change had been performed without complication in 2003.
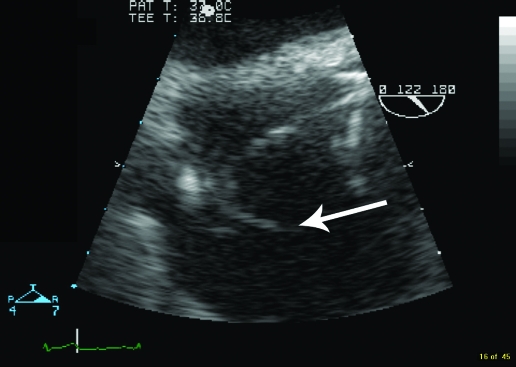
As part of a continuing screen for the source of the sepsis, a further TTE was performed (8 months after the initial investigation) which suggested a vegetation attached to the right ventricular pacing lead; this was confirmed on transoesophageal echocardiography (TOE) (fig 1). On microbiological advice he was placed on an antibiotic regimen of vancomycin, gentamicin and rifampicin, in an effort to cover the usual causes of pacemaker lead infective endocarditis, coagulase negative staphylococci and Staphylococcus aureus. Blood cultures taken at this stage grew Serratia marcescens.
Figure 1.
Transoesophageal echocardiogram showing the vegetation (arrow).
Several days of antibiotics were given while plans were made to extract the device; the inflammatory markers did not settle during this time. Subsequently, the pacemaker device and leads were extracted percutaneously. Cultures taken from the pacing lead tip also grew S marcescens. As the patient was not pacing dependent he was left in a pacemaker-free state. Meropenem and gentamicin were initially commenced while antibiotic sensitivities were awaited. He went on to complete a further 2 week course of oral quinolone antibiotic treatment (ciprofloxacin) according to culture sensitivity. Despite the initial delay in formulating the diagnosis of pacemaker endocarditis, the patient has made an excellent recovery, and remains well 6 months post-procedure. The original pacing indication was for sinus bradycardia; subsequent monitoring has not found this to be profound and because of the severity of the complication a further pacing system has not been implanted, although the patient remains under close follow-up.
Discussion
Right sided endocarditis occurs in 5–10% of all cases of endocarditis. The most common predisposing factors are intravenous drug use and congenital heart disease.1 Endocarditis related to pacemaker lead infection is a rare but serious complication of permanent transvenous pacing. The reported incidence varies considerably from 0.13–7%.2–8
Serratia species are facultatively anaerobic Gram negative bacteria classified in the family Enterobacteriaceae. Along with the other members of the Enterobacteriaceae, they are a rare cause of endocarditis; recent prospective data from the International Collaboration on Endocarditis identified non-HACEK (Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species) Gram negative bacilli as the cause in 49 of over 2700 confirmed cases, of which 0.7 in 1000 were S marcescens. Major risk factors in this series included prosthetic valves and other implanted endovascular material. Intravenous drug use, in contrast to earlier reports which suggested an association with S marcescens endocarditis, was reported in only 4% of cases overall.9
The incidence of non-HACEK Gram negative prosthetic valve endocarditis falls rapidly in the first few months after surgery as endothelialisation occurs. It is a very unusual cause of pacemaker endocarditis. Eighty per cent of infections involving pacemakers are caused by staphylococci, including Staphylococcus aureus and coagulase negative staphylococci. Rarer causes of pacemaker endocarditis include Gram negative bacilli, such as Escherichia coli and Serratia, Pseudomonas, Klebsiella, and Enterobacter species; fungi, such as Candida species and Aspergillus species; and environmental Mycobacteria.10 S marcescens, unlike other members of the Enterobacteriaceae, is an uncommon component of faecal flora; it is, however, found in the environment. Originally considered non-pathogenic, it is now recognised as a major pathogen, particularly in healthcare associated settings, and causes a variety of infections, including bacteraemia. Most cases of bacteraemia are exogenous in origin and urinary catheters and intravenous lines are common portals of entry.10 By the 1970s approximately 20 cases of bacteraemia caused by this species had been identified, of which half had been in the context of previous cardiac valve prosthesis causing endocarditis.11 However, it is very unusual for nosocomial bacteraemia to result in endocarditis and the incidence is not well known. The overall mortality rate of S marcescens bacteraemia ranges from 17–33%, according to epidemiological studies.12
The origin of our patient’s infection remains uncertain. Infection at the time of the pacemaker generator change seems unlikely due to the virulence of the bacteria. He had no suggestion of immunosuppression; the sarcoid had been quiescent for many years and he was not on immunosuppressant medication. No other medical procedures had been undertaken around this time. While several sets of blood cultures were taken during the earlier admissions, empirical antibiotic use before the cultures likely limited the diagnostic yield of this investigation and delayed the diagnosis. This is clearly an important message.
S marcescens carries a chromosomal AmpC β-lactamase. The spontaneous emergence (including emergence on treatment) of derepressed mutants, constitutively expressing high levels of this enzyme, limits the use of many β-lactams for the treatment of infection, including third generation cephalosporins and β-lactam inhibitor combinations such as piperacillin–tazobactam. However, susceptibility to carbapenems is retained. Resistance to other agents, such as aminoglycosides, is variable and may be plasmid borne, hence treatment needs to be tailored according to susceptibilities. Many clinical isolates retain susceptibility to fluoroquinolones, such as ciprofloxacin.
The clinical presentation of pacemaker endocarditis is highly variable. The diagnosis is based on clinical and echocardiographic findings. Previous studies have indicated that the most common presenting clinical and diagnostic features are (1) the presence of a fever (86.5%), (2) elevated C reactive protein (96.2%), and (3) local complications around the pacemaker such as erythema, found in 51.9%.13 The literature suggests that these features generally only fulfil ‘minor’ criteria in the Duke criteria of endocarditis. Moreover splenomegaly, vascular embolic phenomena, and new or changing murmurs are rare in pacemaker endocarditis.14 In essence, an infection of uncertain source in a device patient should prompt consideration of a device related infection. The diagnosis can be very difficult and echocardiography can be diagnostically critical. On occasion device infection may remain presumed even in the absence of clear evidence if no other source is obvious; the clinical judgement of clinicians experienced in device infection may be key.
Some vegetations visualised as mobile masses on echocardiography (particularly small vegetations on the atrial lead) are not infectious, are likely to be fibrinous deposits, and are a normal variant.15 A rabbit model showed that sterile vegetations arose from a catheter implanted within the heart.16 The typical vegetation echocardiographically appears pedunculated, with low echogenicity, and moves out of phase with the structure to which it is attached.17 This is opposed to smaller vegetations that could be myxomatous degeneration of a valve or fibrin deposition. Therefore echocardiographically differentiating masses attached to pacemaker leads can be challenging, often resulting in over estimation of vegetations classified as a major criterion according to the Duke criteria. The yield from TTE showing vegetations in cases of pacemaker endocarditis has been shown to be as low as 22%, in direct comparison to TOE, which demonstrated the presence of lead vegetations or tricuspid vegetations in 96% of cases (p <0.0001).18
The optimal management of pacemaker endocarditis requires antibiotic treatment and the removal of the entire pacing system.6,18 Among 190 patients with pacemaker endocarditis, 12 (41%) of 29 patients treated with antibiotics alone died, compared with 30 (19%) of 161 patients who were treated with antibiotics and surgical removal of the pacemaker system.13 More recent retrospective data perhaps suggest a lower risk of mortality, although the authors acknowledge that lead endocarditis remains a very serious problem.19 Risk factors for death include systemic embolisation, renal impairment, and signs of significant right heart dysfunction.20 The North American Society of Pacing and Electrophysiology (NASPE) classify septicaemia, endocarditis and lead migration as class 1 indications for lead extraction. The duration of drug treatment remains controversial and not well established; therapy is individualised and depends on the site of infection and whether bacteraemia, fever, and echocardiographic evidence of infection are present. Device extraction is a complex and potentially dangerous procedure and is undertaken in only a small number of centres and requires prior planning with surgical backup. Therefore there is often a delay between presentation and device extraction. Before the procedure, antibiotic treatment is initiated to reduce the likely systemic infectious burden created when a vegetation covered lead is removed.
This case highlights pacemaker lead endocarditis, which often remains an extremely elusive entity to categorise, especially when atypical organisms are involved. Patients who have implantable cardiac defibrillators or pacemakers in situ are at risk of this serious, but rare, device complication. It also emphasises the indolent nature the disease process can take. It is imperative that an early assessment is made of device integrity when patients present with infective symptoms where no clear focus is identified. This initially needs to be in the form of a TTE, though as with traditional valve endocarditis, pacemaker endocarditis cannot be excluded after a negative echocardiogram; a TOE may be vital. Treatment with appropriate broad spectrum antibiotics should not be delayed if this diagnosis is suspected, after the drawing of several sets of blood cultures; early discussion with cardiologists, microbiologists and cardiothoracic surgeons is important and early transfer to a specialist centre should be considered.
A further consideration in the management of this case was the previous diagnosis of pulmonary sarcoidosis and the possibility of underlying sarcoid cardiomyopathy. Past echocardiography did not suggest any cardiac involvement and the pulmonary disease had been quiescent for many years. However, a definitive diagnosis of sarcoid cardiomyopathy is often difficult to obtain. Clinical indicators include conduction disease, particularly high degree atrioventricular block and the presence of ventricular dysrhythmias. There was no suggestion of either of these in this case, with the primary indication for pacing being symptomatic sinus bradycardia 16 years before. It would seem likely that if sarcoid cardiomyopathy were the underlying aetiology for the rhythm disturbance, then there would be more evidence of the disease on this presentation. In view of the severity of the complication it was elected to proceed with device explantation, with regular screening for significant conduction disease using prolonged electrocardiographic monitoring of the patient, together with a cardiac structural review using cardiac magnetic resonance imaging. If significant conduction disease was demonstrated, a further pacemaker device would need to be reconsidered.
The fundamental principles behind the management of these cases should be the early removal of the complete device with adjunctive prolonged parental antibiotics (4–6 weeks in duration). Mortality rates with antibiotic treatment alone range from 31–66% in contrast to 13–21%, when complete device removal early followed by prolonged antibiotic treatment is the chosen strategy.13,21 This is based upon the principle that the time taken for the device to be removed shows an inverse relation to survival rates, underlining the importance of early detection with this often insidious problem.10
Learning points
Pacemaker endocarditis remains a rare but potentially life threatening complication of pacemaker implantation.
Pacemaker endocarditis should be considered in patients with a longstanding fever or evidence of septicaemia, where other common causes have been excluded.
Echocardiography, particularly transoesophageal echocardiography, plays a vital role in diagnosing this complication. Obtaining serial blood culture samples, before initiation of antibiotic treatment, remains the mainstay of microbiological identification and potential diagnosis.
Cases of pacemaker endocarditis can be seen many years after initial implantation of the device, where a longstanding fever may be the only salient feature.
Treatment of device infections includes early referral and transfer to a tertiary centre, with early initiation of broad spectrum antibiotics and explantation of the device.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Remetz MS, Quagliarello V. Endovascular infections arising from right-sided heart structures. Cardiol Clin 1992; 10: 137–49 [PubMed] [Google Scholar]
- 2.Conklin EF, Gianelli S, Nealon T. Four hundred consecutive patients with permanent transvenous pacemaker. J Thorac Cardiovasc Surg 1975; 69: 1–7 [PubMed] [Google Scholar]
- 3.Bluhm G. Pacemaker infection: a clinical study with special reference to prophylactic use of some isoxazolyl penicillins. Acta Med Scand Suppl 1985; 699: 1–62 [PubMed] [Google Scholar]
- 4.Block K, Russi E. Right heart endocarditis. Schweiz Med Wochenschr 1989; 47: 1664–72 [PubMed] [Google Scholar]
- 5.Glock Y, Sabatier J, Salvador-Mazencq M, et al. Les endocardites sur électrodes endocavitaires de stimulateurs cardiaques: a propos de 7 cas. Arch Mal Coeur 1986; 79: 483–8 [PubMed] [Google Scholar]
- 6.Loffler S, Kasper J, Postulka J, et al. Septic complications in patients with permanent pacemakers. Cor Vasa 1988; 30: 400–4 [PubMed] [Google Scholar]
- 7.Morgan G, Ginks W, Siddons QH, et al. Septicemia in patients with endocardial pacemaker. Am J Cardiol 1979; 44: 221–4 [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Alvarez J, Duran-Munoz D, Sierra-Quiroga J, et al. Right heart endocarditis and endocardial pacemakers. Ann Thorac Surg 1989; 48: 147–51 [DOI] [PubMed] [Google Scholar]
- 9.Cooper R, Mills J. Serratia endocarditis. A follow up report. Arch Int Med 1980; 140: 199–202 [PubMed] [Google Scholar]
- 10.Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49: 1851–9 [DOI] [PubMed] [Google Scholar]
- 11.Suri RK, Selby AD, et al. Serratia marcescens endocarditis: a report of a case involving Cross-Jones mitral valve prosthesis, with a review of the literature. CMAJ 1971; 104: 1013–4, 106 [PMC free article] [PubMed] [Google Scholar]
- 12.Wisplinghoff H, Bischoff T, Tallent S, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24179 cases from a prospective nationwide surveillance study. Clin Inf Dis 2004; 39: 309–17 [DOI] [PubMed] [Google Scholar]
- 13.Klug D, Lacroix D, Savoye C, et al. Systemic infection related to endocarditis on pacemaker leads clinical presentation and management. Circulation 1997; 95: 2098–107 [DOI] [PubMed] [Google Scholar]
- 14.Arber N, Pras E, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore) 1994; 73: 299–305 [DOI] [PubMed] [Google Scholar]
- 15.Choo MH, Holmes DR, Jr, Gersh BJ, et al. Permanent pacemaker infections: characterization and management. Am J Cardiol 1981; 48: 559–64 [DOI] [PubMed] [Google Scholar]
- 16.Lewis AB, Hayes DL, Holmes DR, Jr, et al. Update on infections involving permanent pacemakers: characterization and management. J Thorac Cardiovasc Surg 1985; 89: 758–63 [PubMed] [Google Scholar]
- 17.Chambers J. Echocardiography in clinical practice. Parthenon publishing, 2002 [Google Scholar]
- 18.Cacoub P, Leprince P, Nataf P, et al. Pacemaker infective endocarditis. Am J Cardiol 1998; 82: 480–4 [DOI] [PubMed] [Google Scholar]
- 19.Catanchin A, Murdock CJ, Athan E. Pacemaker infections: a 10-year experience. Heart Lung Circ 2007; 16: 434–9 [DOI] [PubMed] [Google Scholar]
- 20.Baman TS, Gupta SK, Valle JA, et al. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythmia Electrophysiol 2009; 2: 129–34 [DOI] [PubMed] [Google Scholar]
- 21.Bayer A, Ward J, Ginzton L, et al. Evaluation of new clinical criteria for the diagnosis of infective endocarditis. Am J Med 1994; 96: 211–19 [DOI] [PubMed] [Google Scholar]