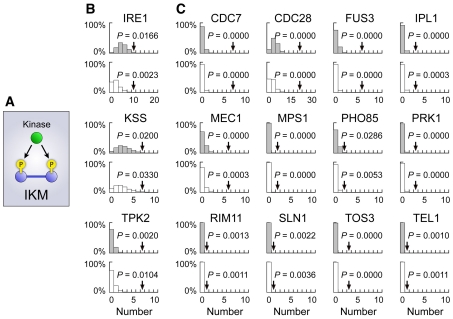
Figure 7. Kinases inferred to yield co-phosphorylation of proteins in the same protein complex.
(A) Conceptual diagram of an interacting kinate module (IKM) motif. (B,C) Kinases revealed to have significantly higher IKM formability than negative controls by data integration of the PPI network with in vitro kinase–substrate relationships (B) and with a literature-based collection of signaling pathways (C). For each kinase, arrows denote number counts of IKMs formed by that kinase and the “whole” (i.e. unfiltered) PPI network, with P values estimated from negative controls of the PPI network generated by RER and NLS (N = 10,000). Expected probability density distributions of number counts of IKMs observed in negative controls generated by node label shuffling and random edge rewiring are shown by gray and white bars, respectively.