Abstract
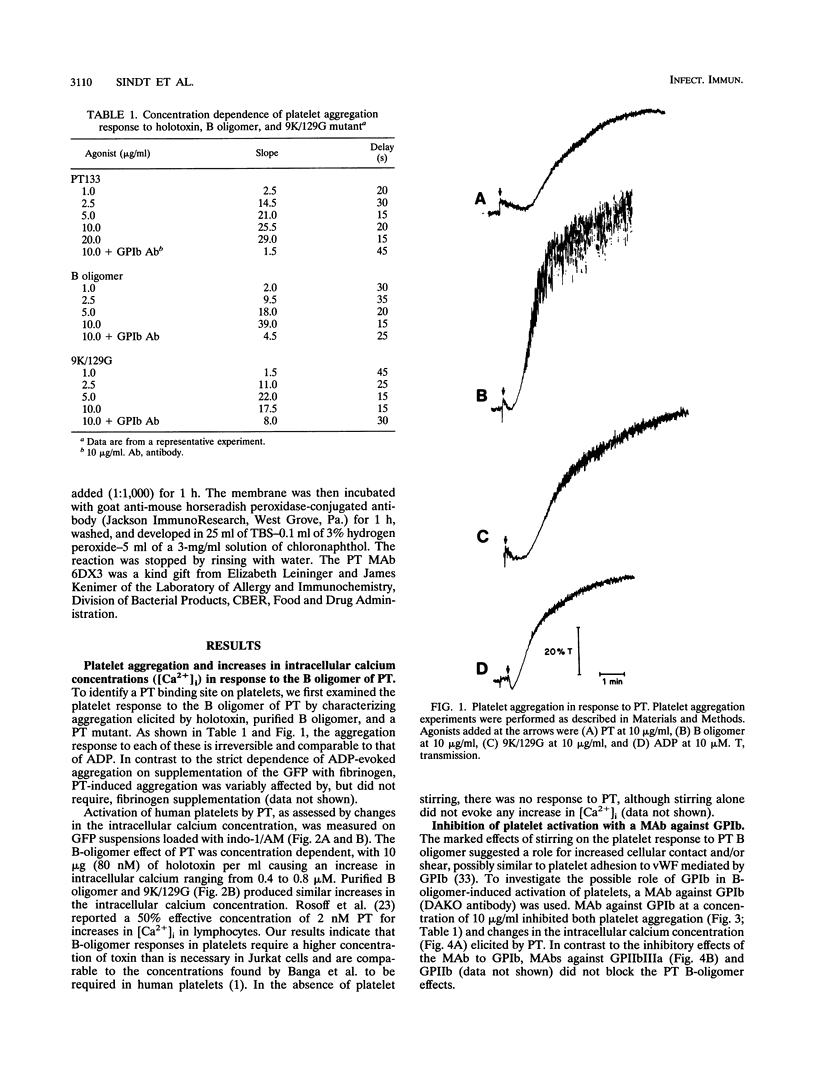
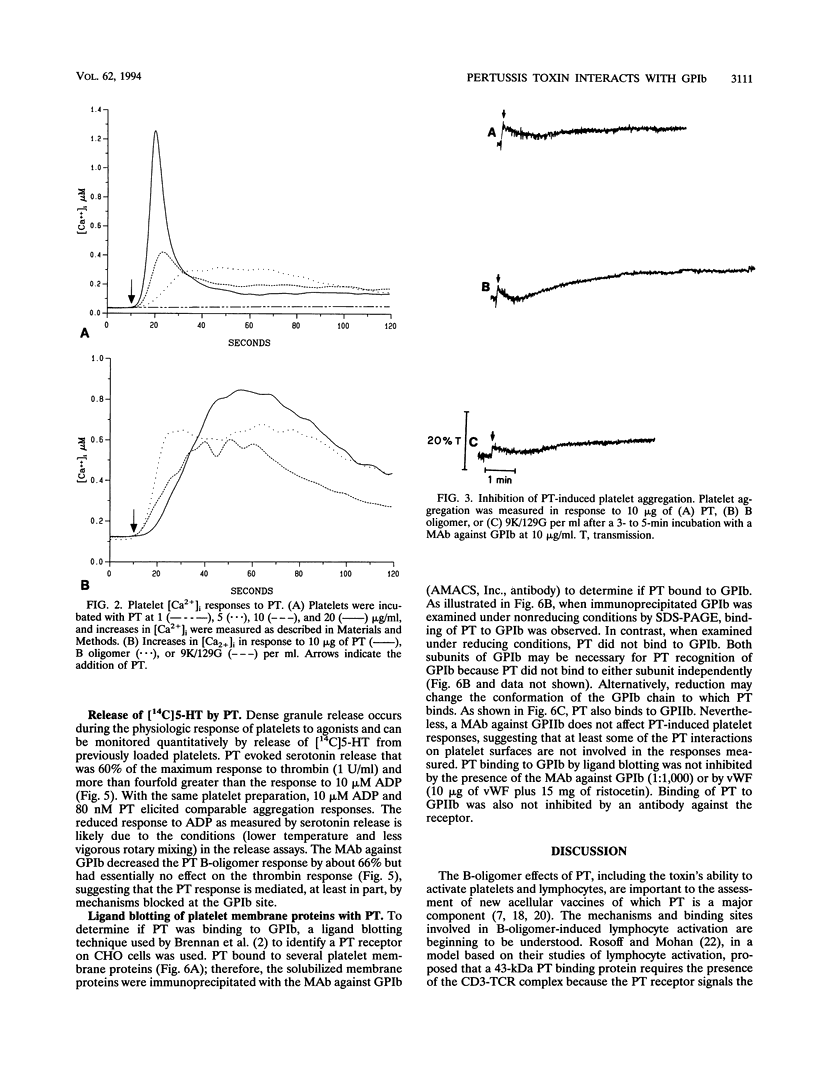
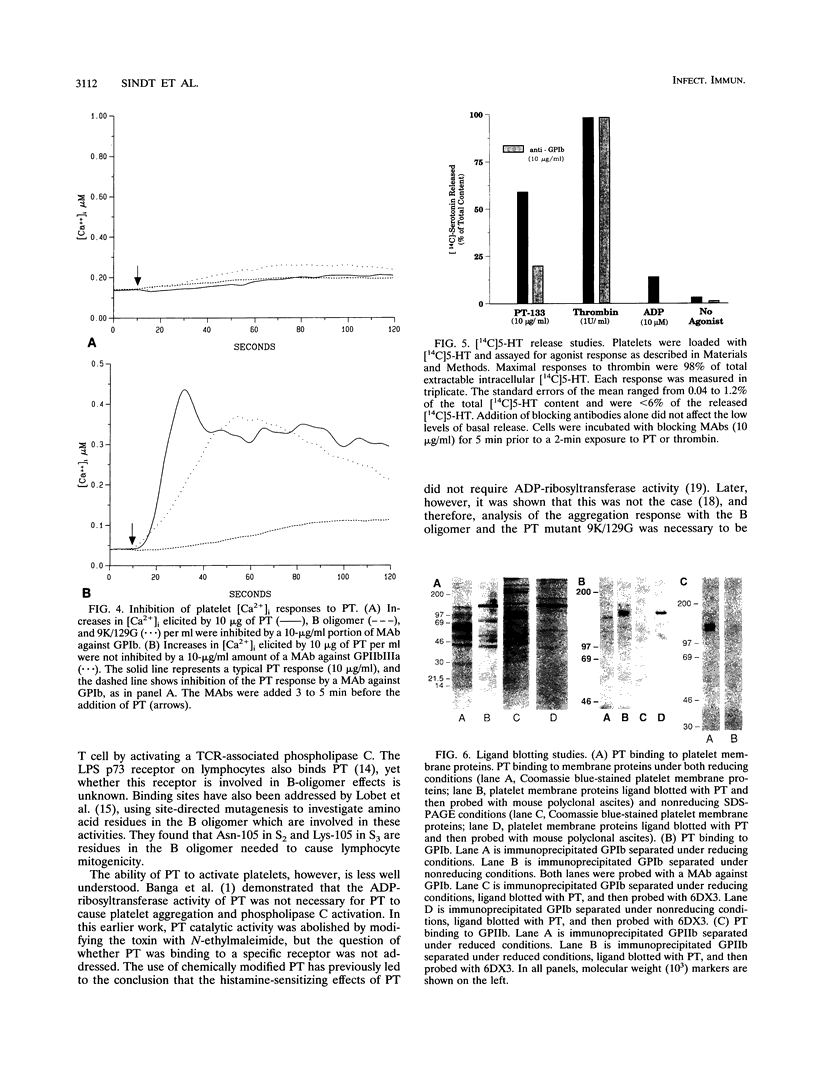
Platelets present a unique model to study the B-oligomer effects of pertussis toxin because they become activated in response to the B oligomer but are not susceptible to ADP-ribosylation by the holotoxin. In these studies, the B oligomer of pertussis toxin caused concentration-dependent platelet activation, as determined by increases in intracellular calcium concentration, dense granule secretion, and platelet aggregation. Stirring was required for pertussis toxin to increase intracellular calcium. A monoclonal antibody against platelet glycoprotein Ib abolished increases in intracellular calcium concentration and increased the latency and reduced the slope of the aggregation response elicited by the B oligomer. Pertussis toxin also evoked [14C]serotonin release from platelets, and this effect was inhibited, though not eliminated, by an antibody against platelet glycoprotein Ib. Binding of pertussis toxin to glycoprotein Ib was observed after nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These data suggest that the B oligomer of pertussis toxin induces platelet activation mediated, at least in part, by an interaction with platelet glycoprotein Ib.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J Biol Chem. 1987 Nov 5;262(31):14871–14874. [PubMed] [Google Scholar]
- Brennan M. J., David J. L., Kenimer J. G., Manclark C. R. Lectin-like binding of pertussis toxin to a 165-kilodalton Chinese hamster ovary cell glycoprotein. J Biol Chem. 1988 Apr 5;263(10):4895–4899. [PubMed] [Google Scholar]
- Britt S. G., Gonias S. L., Sanders J. M., Vandenberg S. R. Agonist and antagonist activities of arylpiperazines at human platelet serotonin2 receptors. J Pharmacol Exp Ther. 1988 Dec;247(3):965–970. [PubMed] [Google Scholar]
- Burns D. L., Kenimer J. G., Manclark C. R. Role of the A subunit of pertussis toxin in alteration of Chinese hamster ovary cell morphology. Infect Immun. 1987 Jan;55(1):24–28. doi: 10.1128/iai.55.1.24-28.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. G., Armstrong G. D. Lymphocyte receptors for pertussis toxin. Infect Immun. 1990 Dec;58(12):3840–3846. doi: 10.1128/iai.58.12.3840-3846.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Edwards K. M. Acellular pertussis vaccines--a solution to the pertussis problem? J Infect Dis. 1993 Jul;168(1):15–20. doi: 10.1093/infdis/168.1.15. [DOI] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Handa M., Kamata T., Kawano K., Kawai Y., Watanabe K., Kawakami K., Sakai K., Fukuyama M., Itagaki I. Transmembrane calcium influx associated with von Willebrand factor binding to GP Ib in the initiation of shear-induced platelet aggregation. Thromb Haemost. 1993 May 3;69(5):496–502. [PubMed] [Google Scholar]
- Kieffer N., Phillips D. R. Platelet membrane glycoproteins: functions in cellular interactions. Annu Rev Cell Biol. 1990;6:329–357. doi: 10.1146/annurev.cb.06.110190.001553. [DOI] [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Evidence that lipopolysaccharide and pertussis toxin bind to different domains on the same p73 receptor on murine splenocytes. Infect Immun. 1993 Apr;61(4):1359–1364. doi: 10.1128/iai.61.4.1359-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet Y., Feron C., Dequesne G., Simoen E., Hauser P., Locht C. Site-specific alterations in the B oligomer that affect receptor-binding activities and mitogenicity of pertussis toxin. J Exp Med. 1993 Jan 1;177(1):79–87. doi: 10.1084/jem.177.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Macintyre E. A., Tatham P. E., Abdul-Gaffar R., Linch D. C. The effects of pertussis toxin on human T lymphocytes. Immunology. 1988 Jul;64(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Nencioni L., Pizza M. G., Volpini G., De Magistris M. T., Giovannoni F., Rappuoli R. Properties of the B oligomer of pertussis toxin. Infect Immun. 1991 Dec;59(12):4732–4734. doi: 10.1128/iai.59.12.4732-4734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Pizza M., Covacci A., Bartoloni A., Perugini M., Nencioni L., De Magistris M. T., Villa L., Nucci D., Manetti R., Bugnoli M. Mutants of pertussis toxin suitable for vaccine development. Science. 1989 Oct 27;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- Rogers T. S., Corey S. J., Rosoff P. M. Identification of a 43-kilodalton human T lymphocyte membrane protein as a receptor for pertussis toxin. J Immunol. 1990 Jul 15;145(2):678–683. [PubMed] [Google Scholar]
- Rosoff P. M., Mohan C. Unidirectional, heterologous desensitization of the pertussis toxin receptor by the CD3/TCR complex. J Immunol. 1992 Nov 15;149(10):3191–3199. [PubMed] [Google Scholar]
- Rosoff P. M., Walker R., Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987 Oct 1;139(7):2419–2423. [PubMed] [Google Scholar]
- Savage B., Shattil S. J., Ruggeri Z. M. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin alpha IIb beta 3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J Biol Chem. 1992 Jun 5;267(16):11300–11306. [PubMed] [Google Scholar]
- Sommermeyer H., Resch K. Pertussis toxin B-subunit-induced Ca2(+)-fluxes in Jurkat human lymphoma cells: the action of long-term pre-treatment with cholera and pertussis holotoxins. Cell Signal. 1990;2(2):115–128. doi: 10.1016/0898-6568(90)90015-3. [DOI] [PubMed] [Google Scholar]
- Stewart S. J., Prpic V., Johns J. A., Powers F. S., Graber S. E., Forbes J. T., Exton J. H. Bacterial toxins affect early events of T lymphocyte activation. J Clin Invest. 1989 Jan;83(1):234–242. doi: 10.1172/JCI113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad C. F., Carchman R. A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987 Dec 10;225(1-2):16–20. doi: 10.1016/0014-5793(87)81123-7. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- VandenBerg S. R., Gonias S. L. Covalent complexes of albumin with serotonin, ketanserin and lysergic acid antagonize the activity of serotonin in human platelets. Life Sci. 1989;44(23):1777–1785. doi: 10.1016/0024-3205(89)90565-1. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate--dependent decrease of adhesion in von Willebrand's disease and the Bernard-Soulier syndrome. J Lab Clin Med. 1978 Nov;92(5):750–764. [PubMed] [Google Scholar]
- van't Wout J., Burnette W. N., Mar V. L., Rozdzinski E., Wright S. D., Tuomanen E. I. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect Immun. 1992 Aug;60(8):3303–3308. doi: 10.1128/iai.60.8.3303-3308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



