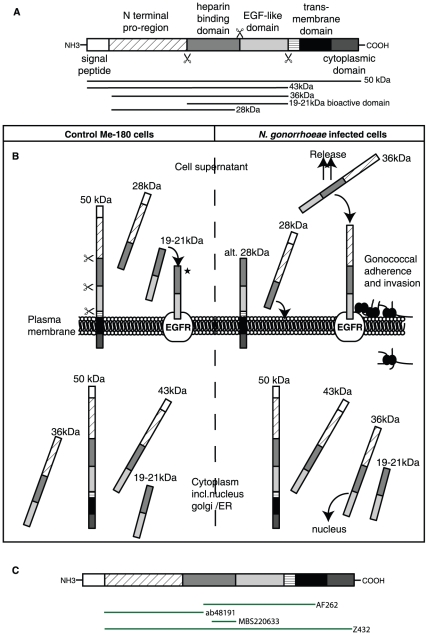
Figure 7. N. gonorrhoeae induces alternative processing and release of amphiregulin.
(A) Pro-amphiregulin is synthesized by 252 amino acids (50 kDa). The protein consists of a signal peptide, a N-terminal pro-region, a heparin binding domain, an EGF-like domain, a transmembrane domain, and a cytoplasmic domain. Due to metalloprotease dependent cleavage, amphiregulin can produce several products with the molecular sizes 50, 43, 36, 28, and 19–21 kDa. (B) Schematic illustration of amphiregulin expression, cleavage pattern and processing in Me-180 cells. N. gonorrhoeae infection changes the cleavage pattern, subcellular localization, processing, and release of amphiregulin in Me-180 cells. (* Indicates alternative fate of cleaved amphiregulin [11].) (C) Illustration of the recognition sites of the antibodies used in this study.