Fig. 4.
The C terminus is required to protect the 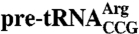 anticodon stem from kethoxal. (A) 32P-labeled wild-type
anticodon stem from kethoxal. (A) 32P-labeled wild-type  was incubated with the indicated concentrations of Lhp1p (left) or Lhp1p(1-227) (right) and fractionated in native gels to separate RNAs from RNPs. Dissociation constants (± s.d.) were derived from three independent experiments. As described for Lhp1p (21), upon addition of increasing protein, both proteins form complexes that migrate with slower mobility. As these complexes are preferentially dissociated by competitor RNAs, they may represent binding by a second protein molecule to a less specific site on the pre-tRNA (21). (B) Unlabeled
was incubated with the indicated concentrations of Lhp1p (left) or Lhp1p(1-227) (right) and fractionated in native gels to separate RNAs from RNPs. Dissociation constants (± s.d.) were derived from three independent experiments. As described for Lhp1p (21), upon addition of increasing protein, both proteins form complexes that migrate with slower mobility. As these complexes are preferentially dissociated by competitor RNAs, they may represent binding by a second protein molecule to a less specific site on the pre-tRNA (21). (B) Unlabeled  was incubated in the absence (lanes 1–3) or presence of Lhp1p(1-227) (lanes 4–6) or Lhp1p (lanes 7–9). Kethoxal was added and the reaction incubated an additional 15 min at the indicated temperature. Sites of modification were detected by primer extension; thus, no information was obtained about the 3′ end of the RNA. Additional bands which do not correspond to Gs may be due to degradation or secondary structure. (C) Sites of kethoxal modification on
was incubated in the absence (lanes 1–3) or presence of Lhp1p(1-227) (lanes 4–6) or Lhp1p (lanes 7–9). Kethoxal was added and the reaction incubated an additional 15 min at the indicated temperature. Sites of modification were detected by primer extension; thus, no information was obtained about the 3′ end of the RNA. Additional bands which do not correspond to Gs may be due to degradation or secondary structure. (C) Sites of kethoxal modification on  are indicated by arrowheads. (D–F) PhosphorImager quantitation of anticodon stem modifications in the presence of no protein (D), Lhp1p(1-227) (E), or full-length Lhp1p (F).
are indicated by arrowheads. (D–F) PhosphorImager quantitation of anticodon stem modifications in the presence of no protein (D), Lhp1p(1-227) (E), or full-length Lhp1p (F).

