Fig. 3.
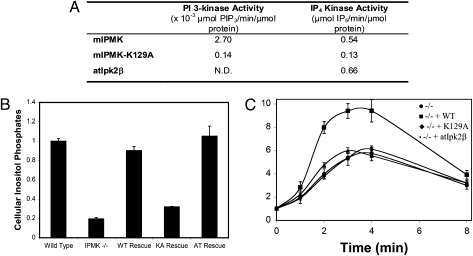
The PI3K activity of IPMK is required for full activation of Akt in response to EGF. (A) Comparison of in vitro specific activities of mouse IPMK, mouse IPMK-K129A, and atIpk2β. His-tagged recombinant proteins were purified from Escherichia coli, as previously described for IP6K1 (35). All reactions were monitored over time and specific activities were calculated based on the linear range of the reaction curves. (B) Wild-type IPMK, and atIpk2β fully restore higher inositol phosphate production in IPMK−/− MEFs, but IPMK-K129A does not. Wild-type IPMK, IPMK-K129A, and atIpk2β were stably expressed in IPMK−/− MEFs. Equal numbers of each cell type were labeled to with [3H]myo-inositol. After extraction, inositol phosphates were resolved by HPLC and quantified by scintillation counting. Data represent the sums of cellular IP5, IP6, and IP7, and are means of three independent experiments. Error bars represent SEs. (C) Wild-type IPMK restores EGF-induced Akt phosphorylation in IPMK−/− MEFs, but IPMK-K129A and atIpk2β do not. Equal numbers of the same stable cell lines used in the experiment shown in B were plated, serum-starved overnight, stimulated with EGF, and lysed. Lysates were analyzed for phospho-Akt-T308, total Akt, and myc-IPMK expression by immunoblotting. Data are means of three independent experiments and error bars are SEs. For representative raw data, see Fig. S5.