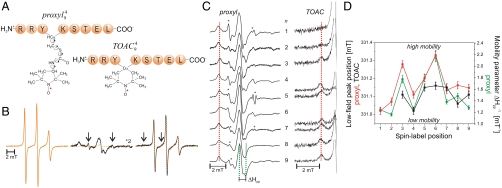
Fig. 1.
Mobility of spin-labeled peptides bound to TAP. (A) Structure of 9-mer peptides modified by proxyl or TOAC spin probes at position 4. Arrows indicate the possible rotations around different bonds in the proxyl spin probe. (B) Spin-labeled 9-mer peptides bind specifically to TAP. Spin-normalized CW derivative spectra of 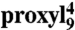 free in solution (orange line), bound to TAP (black line, multiplied by two for clarity, 10 μM
free in solution (orange line), bound to TAP (black line, multiplied by two for clarity, 10 μM 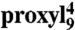 after incubation with 48 μM active TAP at 277 K for 15 min), and competed with a 165-fold molar excess of unlabeled competitor peptide RRYQKSTEL for 2 min at 310 K. The spectrum of free peptide is scaled (dotted orange line) and superimposed to the spectra of bound and competed spin-labeled peptides to reveal the fraction of free peptide or residual bound peptide after competition, respectively (indicated by arrows). (C) EPR derivative spectra of bound proxyl and TOAC-labeled peptides. Peptides were incubated with TAP for 15 min at 4 °C. For proxyl-labeled peptides, the fraction of free peptide was subtracted. Asterisks indicate small artifacts due to subtraction. The red dotted line highlights displacement of the low-field peak, and the green dotted lines show an exemplary central line width (ΔHpp). (D) Mobility parameters extracted from the spectra shown in panel C versus residue numbers: the low-field peak positions for the proxyl and TOAC probes are shown in red and black, respectively; the inverse of the line width is shown only for the proxyl probes in green.
after incubation with 48 μM active TAP at 277 K for 15 min), and competed with a 165-fold molar excess of unlabeled competitor peptide RRYQKSTEL for 2 min at 310 K. The spectrum of free peptide is scaled (dotted orange line) and superimposed to the spectra of bound and competed spin-labeled peptides to reveal the fraction of free peptide or residual bound peptide after competition, respectively (indicated by arrows). (C) EPR derivative spectra of bound proxyl and TOAC-labeled peptides. Peptides were incubated with TAP for 15 min at 4 °C. For proxyl-labeled peptides, the fraction of free peptide was subtracted. Asterisks indicate small artifacts due to subtraction. The red dotted line highlights displacement of the low-field peak, and the green dotted lines show an exemplary central line width (ΔHpp). (D) Mobility parameters extracted from the spectra shown in panel C versus residue numbers: the low-field peak positions for the proxyl and TOAC probes are shown in red and black, respectively; the inverse of the line width is shown only for the proxyl probes in green.