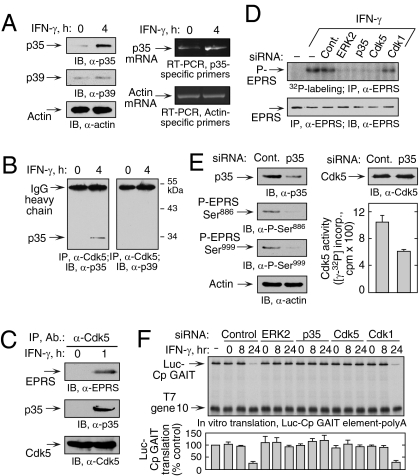
Fig. 2.
Induction of p35 activator protein by IFN-γ is required for Ser886 phosphorylation by Cdk5. (A) IFN-γ induces p35 activator protein. (Left) Lysates from U937 cells treated with IFN-γ for 4 h were immunoblotted. (Right) p35 and β-actin mRNA was determined by RT-PCR of total cellular RNA. (B) IFN-γ induces Cdk5 binding to p35. Lysates from U937 cells treated with IFN-γ for 4 h were coimmunoprecipitated with anti-Cdk5 antibody and probed with anti-p35 (Left) and anti-p39 (Right) antibodies. (C) Cdk5/p35 phosphorylates EPRS at Ser886. Lysates from U937 cells treated with IFN-γ for 1 h were immunoprecipitated and immunoblotted as shown. (D) Knockdown of p35 blocks EPRS phosphorylation. (Upper) siRNAs were transfected into U937 cells and incubated with 32P-orthophosphate and IFN-γ for 4 h. Lysates were immunoprecipitated with anti-EPRS antibody, and EPRS phosphorylation was determined by autoradiography. (Lower) Total EPRS was detected with anti-EPRS antibody. (E) Knockdown of p35 activator protein blocks two-site EPRS phosphorylation. U937 cells were transfected with siRNA against p35 and then treated with IFN-γ as in Fig. 1D. (Left) Knockdown efficiency and EPRS phosphorylation were determined by immunoblot analysis.(Right) Lysates were incubated with anti-Cdk5 antibody, and precipitates were used for in vitro phosphorylation of Cdk5-specific peptide substrate. Aliquots were spotted on phosphocellulose P-81, and the incorporation of 32P into peptide was determined by scintillation counting (mean ± SEM; n = 3 experiments). (F) ERK2, p35, and Cdk5 are required for GAIT complex–mediated translational suppression. U937 cells were transfected with the siRNAs shown and incubated with IFN-γ for up to 24 h. Lysates were added to RRL for in vitro translation of capped, polyadenylated luciferase (Luc) reporter transcript bearing the Cp GAIT element in the presence of 35S-Met. T7 gene 10 RNA lacking the GAIT element was cotranslated as a specificity control. Luc translation was quantified by densitometry, normalized by T7 gene 10, and expressed as percentage of control (mean ± SEM; n = 3 experiments).