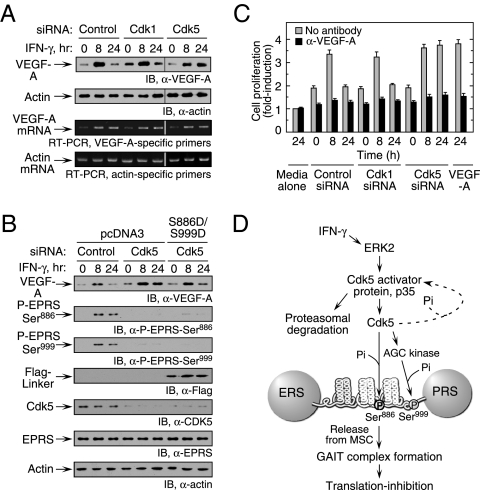
Fig. 5.
Cdk5-mediated EPRS phosphorylation regulates VEGF-A expression. (A) Inhibition of Cdk5 expression enhances VEGF-A expression. U937 cells were transfected with siRNA as shown and then incubated with IFN-γ for up to 24 h. (Upper) Lysates were probed with anti–VEGF-A or actin antibodies. (Lower) RT-PCR analysis of total cellular RNA was performed using gene-specific primers. (B) Expression of phosphomimetic EPRS linker in Cdk5 knockdown cells restores inhibition of VEGF-A expression. U937 cells cotransfected with siRNAs and Flag-tagged, double-phosphomimetic EPRS linker (or vector control) were incubated with IFN-γ for up to 24 h. VEGF-A, Cdk5, endogenous EPRS phosphorylation, Flag-tagged EPRS linker, and actin loading control were determined by immunoblot analysis. (C). U937 cell Cdk5 activity influences VEGF-A–directed EC proliferation. Conditioned medium from siRNA-transfected and IFN-γ–treated U937 cells was added to bovine aortic ECs. ECs treated with recombinant VEGF-A (10 ng/mL) served as a positive control. Proliferation was determined by an MTT assay and expressed as fold induction compared with ECs treated with medium alone (gray bars). The activity of conditioned medium preincubated with anti–VEGF-A antibody was assessed as well (black bars). Values shown are mean ± SEM (n = 3 experiments in triplicate). (D) Schematic of IFN-γ–induced signaling events that activate Cdk5/p35 to induce EPRS phosphorylation and suppress gene expression by translation control.