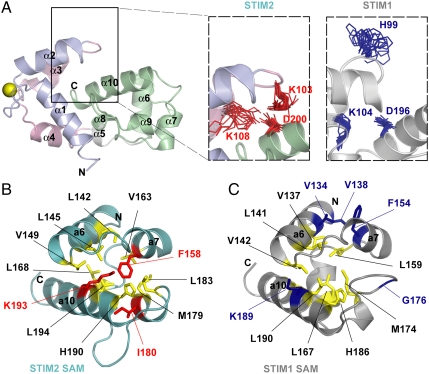
Fig. 3.
(A) Relative positions of STIM2 Lys103, Lys108, and Asp200 compared to aligned STIM1 His99, Lys104, and Asp196. The charged side chain positions of the 20 lowest energy structures for STIM2 are shown in red; the aligned residues of the 20 lowest energy structures for STIM1 are in blue. (B) Hydrophobic side chain packing in the STIM1 and STIM2 SAM domains. Residues in the hydrophobic cores are colored in yellow. The Lys193, Ile180, and Phe158 which enhance the STIM2 SAM domain (teal) hydrophobic core are shown in red. The aligned STIM1 SAM residues (i.e., Lys189, Gly176, and Phe154) as well as Val134 and Val138 which pack against Phe154 are in blue.