Abstract
Keratin 8 (K8) is a major intermediate filament protein present in enterocytes and serves an antiapoptotic function in hepatocytes. K8-null mice develop colonic hyperplasia and colitis that are reversed after antibiotic treatment. To investigate the pathways that underlie the mechanism of colonocyte hyperplasia and the normalization of the colonic phenotype in response to antibiotics, we performed genome-wide microarray analysis. Functional annotation of genes that are differentially regulated in K8−/− and K8+/+ isolated colon crypts (colonocytes) identified apoptosis as a major altered pathway. Exposure of K8−/− colonocytes or colon organ (“organoid”) cultures, but not K8−/− small intestine organoid cultures, to apoptotic stimuli showed, surprisingly, that they are resistant to apoptosis compared with their wild-type counterparts. This resistance is not related to inflammation per se because T-cell receptor α-null (TCR-α−/−) and wild-type colon cultures respond similarly upon induction of apoptosis. Following antibiotic treatment, K8−/− colonocytes and organ cultures become less resistant to apoptosis and respond similarly to the wild-type colonocytes. Antibiotics also normalize most differentially up-regulated genes, including survivin and β4-integrin. Treatment of K8−/− mice with anti–β4-integrin antibody up-regulated survivin, and induced phosphorylation of focal adhesion kinase with decreased activation of caspases. Therefore, unlike the proapoptotic effect of K8 mutation or absence in hepatocytes, lack of K8 confers resistance to colonocyte apoptosis in a microflora-dependent manner.
Keratins exist as obligate noncovalent heteropolymers of type I (K9–K28) and type II (K1–K8 and K71–K80) proteins and make up the intermediate filament (IF) cytoskeleton of epithelial cells (1–3). In adult hepatocytes, the IF network consists of simple epithelial K8 and K18, whereas in the intestine the network consists of K7, K8, K18, K19, and K20, with K8 being the major type II keratin (2, 4, 5). Absence or mutation of K8 or K18 renders hepatocytes markedly susceptible to apoptosis, and in humans and mice K8 and K18 mutations predispose their carriers to acute and chronic end-stage liver disease and liver disease progression (5–8). In addition to the effects of keratins in the liver, K8−/− mice develop colonic hyperplasia and chronic spontaneous colitis (9, 10) that is amenable to early treatment with broad-spectrum antibiotics (11).
Although hepatocytes in K8−/− and K18−/− mice are highly sensitive to apoptotic stimuli (5, 12, 13), K18−/− intestine appears normal (14), probably reflecting the functional redundancy of additional type I keratins in the intestine (4, 15). In humans, the association of K8 variants with inflammatory bowel disease (IBD) is unclear (16, 17). The differences between the liver- and intestine-proliferative phenotypes and the associations (or lack thereof) with human disease highlight the potential importance of the microenvironment and cell-specific modifiers.
In contrast to the findings in K8−/− hepatocytes, we show in this study that K8−/− colonocytes, but not K8−/− small intestine enterocytes or T-cell receptor α-null (TCRα−/−) colonocytes, are relatively resistant to apoptosis, but this resistance can be reversed in K8−/− mice by antibiotic treatment. In addition, we show that K8−/− colonocytes up-regulate survivin and β4-integrin, with the latter playing an important role in enterocyte anoikis (18, 19). The keratin IF network links to β4-integrin at the site of hemidesmosomes via interaction with the cytoskeletal linker protein plectin and BP180 (20, 21). Furthermore, we provide a mechanism for the altered susceptibility to apoptosis, because in vivo treatment of K8−/− mice with anti–β4-integrin antibody further up-regulates survivin, leads to the activation of down-stream phosphorylation of focal adhesion kinase (FAK), and decreases caspase activation. The resistance to apoptosis observed at the tip of the colonic crypt coupled with colonocyte proliferation along most of the crypt likely contribute to the observed colonic hyperproliferation in K8−/− mice.
Results
Expression Profiling in K8−/− and K8+/+ Colonocytes Shows Apoptosis Is a Prominent Keratin-Regulated Biological Pathway.
To understand better the effect of K8 absence on the colonic hyperplasia and colitis phenotype and the normalization of this phenotype following antibiotic treatment (11), we sought to identify colonocyte genes that are differentially expressed in response to the absence of K8 or to suppression of luminal bacteria. Microarray analysis revealed that several hundred genes were differentially regulated in K8+/+ and K8−/− primary isolated mouse colonocytes (Fig. 1A). The 20 genes most highly up-regulated in the K8−/− colon crypts are listed in Table S1. Antibiotic pretreatment of the animals led to near-complete normalization of the differentially altered genes in the K8−/− colon (Fig. 1B). The antibiotics used herein effectively suppressed the presence of luminal bacteria as determined by stool bacterial DNA content (Table S2). Using real-time PCR, we validated several of the genes that manifested marked up- or down-regulation in the microarray findings (Table S3). Analysis using the Jubilant PathArt database package Physiology software revealed that apoptosis was the most differentially regulated biological pathway, with 46 of 160 apoptosis pathway genes showing significant differential expression (P = 3 × 10−5) in K8−/− and K8+/+ colonocytes (Table S4). The software identified growth and differentiation as the second most significant pathway, with 39 of 135 genes differentially expressed in K8−/− and K8+/+ colonocytes (P = 1 × 10−4).
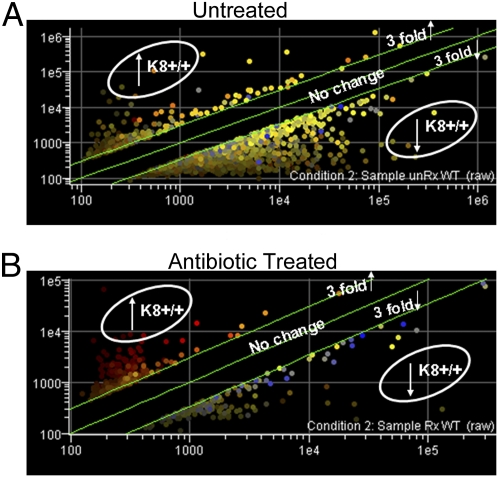
Fig. 1.
Gene expression profiles of K8−/− and K8+/+ colonocytes show decreased numbers of differentially regulated genes after antibiotic treatment. (A) Total RNA from freshly isolated K8−/− and K8+/+ colonocytes was used for microarray analysis. The graph depicts results from GeneSpring analysis. Upper and lower green lines represent genes threefold up- or down-regulated in K8+/+ compared with K8−/− colonocytes. (B) An analysis to that similar in A except that the RNA was isolated from colonocytes of antibiotic-treated K8−/− and K8+/+ mice. Note the marked decrease in differential expression between K8−/− and K8+/+ colonocyte genes following antibiotic treatment. Two hundred eighty-eight genes were threefold up-regulated, and 460 genes were threefold down-regulated in K8+/+ compared with K8−/− colonocytes, but after antibiotic treatment only 189 genes and 290 genes remained threefold up-regulated and down-regulated, respectively.
K8−/− Colonocytes Overexpress Survivin and, Unlike K8−/− Cells from the Small Intestine and K8−/− Colonocytes from Antibiotic-Treated Mice, Are Resistant to Apoptosis.
In the human small intestine, it is estimated that 1010 cells are shed per day; despite the large number of cells being shed, the epithelial barrier is maintained (22). Although the signals that initiate cell shedding are not known (22), enterocytes undergo a spontaneous form of apoptosis, termed “anoikis,” upon detachment from the cellular matrix (18). Because of the established predisposition of K8−/− (and K8, K18 mutant) hepatocytes and livers to mechanical and nonmechanical injury and to apoptosis (23–28), we compared the apoptotic response of K8−/− and K8+/+ small intestine and colons. K8−/− colonic crypts (here called “colonocytes”) were isolated in parallel, and cleavage/activation of caspases was assessed as an indicator of apoptosis. Surprisingly, and unlike K8−/− hepatocytes, the K8−/− colonocytes are resistant to apoptosis based on the relative decrease in cleavage of caspase-3, -7, and -9 (Fig. 2A). Activation of apoptosis also was associated with K8 S80 phosphorylation in K8+/+ colonocytes (Fig. 2A), and ablation of S80A in hepatocytes by an S80A mutation leads to marked predisposition to apoptosis upon stimulation with Fas ligand (29). Similar findings were noted using whole-organ fragments (here called “organoids”) of the colon (Fig. 2B), but not of the small intestine (Fig. 2C), that were kept in culture for 1 h. Notably, small intestine organoids from K8−/− mice appear to be more predisposed to apoptosis as compared to K8+/+ small intestine based on the enhanced cleavage of caspase-7 (Fig. 2C). Consistent with susceptibility to apoptosis after 1 h culture, K18 fragmentation (K18 Asp237) (30) was detected in both colon and small intestine organoid K8+/+ cultures (Fig. 2 B and C). To ensure that caspase activation involved colonocytes rather than lamina propria or other nonepithelial cells, we analyzed cleaved caspase-7 in frozen colon pieces from the organ cultures by immunofluorescence staining (Fig. 2D). A significant increase in apoptotic cells was seen at the luminal or surface end of the K8+/+ colonic crypts, compared with K8−/− colonic crypts, and the cells were identified as colonocytes by their coexpression of K19 (Fig. 2D a and b).
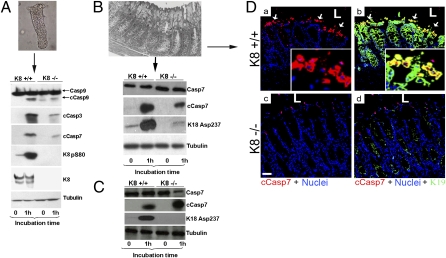
Fig. 2.
Primary colonocytes and colon but not small intestine organ cultures from K8−/− mice are less susceptible to apoptosis than parallel cultures from K8+/+ mice. (A) K8+/+ and K8−/− colonocytes were cultured in medium for 1 h. Cell homogenates then were analyzed by immunoblotting using antibodies to cleaved caspases (cCasp) or total caspases (Casp), K8 pS80, K8, and tubulin. (B) Colon and (C) small intestine organoids were cultured and then analyzed as in A. (D) Frozen sections from K8+/+ (a and b) and K8−/− (c and d) 1-h colon primary organ cultures were triple stained for anti–cleaved caspase-7 red), anti-K19 (green), and nuclei (blue). Merged images are shown in b and d. Arrows point to apoptotic cells at the luminal (L) tips of K8+/+ crypts. Insets show magnified views. (Scale bar in c: 50 μm.)
We then used known inducers of apoptosis such as staurosporine and Fas antibody to examine potential differential sensitivity to apoptosis of K8−/− and K8+/+ colonocytes. Similar to findings noted during colonocyte isolation (Fig. 2), K8−/− colons are more resistant than K8+/+ colons to the induction of apoptosis by both staurosporine and Fas. This finding was confirmed biochemically by the limited formation of cleaved caspase-7 (Fig. 3A) and by immunofluorescence staining (Fig. 3B). In addition, the resistance of K8−/− colonocytes to apoptosis, compared with K8+/+ colonocytes, was confirmed using TUNEL staining (Fig. S1). Of note, and consistent with the “normalization” of the differentially regulated genes in K8−/− and K8+/+ colonocytes in antibiotic-treated mice, K8−/− colonocyte resistance to apoptosis was reversed with antibiotic pretreatment (Fig. 3 C and D).
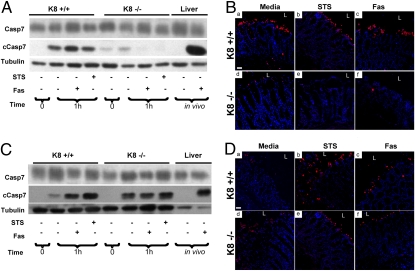
Fig. 3.
Antibiotic treatment of mice reverses the K8−/− resistance to apoptosis. Colon organ cultures from K8 mice not treated (A and B) or treated with antibiotic (C and D) were maintained in the presence or absence of staurosporine (STS) or Fas for 1 h. Livers from FVB/n mice treated with Fas antibody (i.p. administration) for 4 h or not treated were included as positive and negative controls, respectively, for formation of cleaved caspase-7 (cCasp7). The organoid cultures then were processed for immunoblotting (A and C) or antibody staining (B and D). Antibody staining was done for anti–cleaved caspase-7 (red) and nuclei (blue). Note the resistance to apoptosis in colons from K8−/− mice not treated with antibiotic (A and images d–f in B) and the reversal of this resistance to apoptosis in colons from K8−/− mice treated with antibiotics (C and images d–f in D). Casp7, caspase 7; L, lumen.
It is difficult to study the relationship of microflora to the colonic hyperplasia in the absence of inflammation, because treatment with antibiotics reverses both the inflammatory response and the colonic hyperplasia in K8−/− mice (11). To assess whether inflammation per se or inflammation triggered by microbes contributes to the observed alteration in apoptosis, we examined the differences in colonocyte apoptosis in another chronic colitis model, namely the TCRα−/− model of chronic colitis (31). Both TCRα−/− and K8−/− mice manifest a T-helper type 2 (Th2) type of colitis (11, 31). Unlike the situation in K8−/− colon (Fig. 3 A and B), TCRα−/− colonocytes were not resistant to apoptosis compared with their wild-type counterparts (Fig. S2). In agreement with the histologically evident K8−/− colonic hyperproliferation, Ki67 staining was increased in K8−/− compared with K8+/+ colonocytes (Fig. 4A). Quantification showed a significant increase in the number of Ki67+ cells present in K8−/− colonic crypts, even when normalized to crypt length (0.12 μm−1 vs. 0.05 μm−1, P < 0.001). As expected, most of the Ki67+ cells are present at the bottom half of the crypt, but they can be found in the lower three-fourths of the crypt. Consistent with the findings from Ki67 staining, immune blotting (9) shows increased numbers of proliferating cell nuclear antigen (PCNA)-positive cells in K8−/− compared with K8+/+ colons.
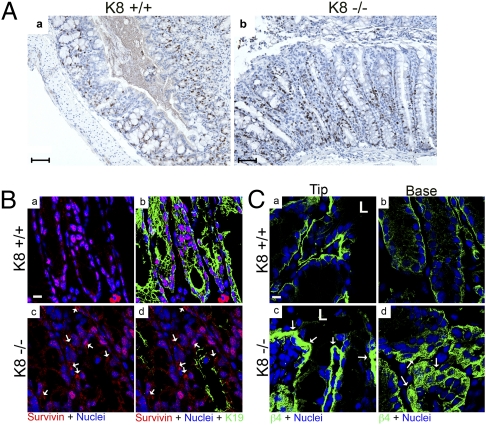
Fig. 4.
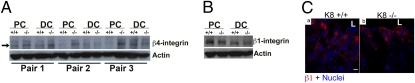
Altered proliferation and expression of survivin and β4-integrin in K8−/− colon. (A) Paraffin-embedded colon sections from K8+/+ and K8−/− mice stained with Ki67 demonstrate increased colonocyte proliferation in K8−/− compared with K8+/+ mice. (Scale bar: 10 μm.) An average of 70–100 crypts per colon was analyzed. Quantification showed a significant increase in the number of Ki67+ cells in K8−/− colonic crypts, normalized to crypt length (0.12 μm−1 versus 0.05 μm−1). P < 0.001, when comparing K8+/+ versus K8−/−; n = 3 pairs. (B) Frozen colon sections from 3-mo-old K8+/+ (a and b) and K8−/− (c and d) mice were triple stained for survivin (red), K19 (green), and nuclei (blue). Merged images for the indicated double or triple staining are shown. Increased survivin, especially in the cytoplasm, was seen in K8−/− colons (arrows in c and d). (C) Frozen colon sections (crypt tips, Left; bases, Right) from K8+/+ (a and b) and K8−/− (c and d) mice were double stained for β4-integrin (green), and nuclei (blue). Note increased β4-integrin staining (arrows) in the basolateral compartment of K8−/− epithelial cells (c and d). (Scale bar: 10 μm.) L, lumen.
Given the observed decreased susceptibility of K8−/− colonocytes to apoptosis, we used the microarray data to survey the antiapoptosis genes that were differentially regulated. Among the differentially regulated genes, survivin levels increased in K8−/− colonocytes by 3.6-fold with microarray analysis and by 5.7-fold with quantitative PCR (qPCR) analysis (Table S3). Survivin is a member of the inhibitor of apoptosis family with a dual role based on its localization: nuclear survivin is involved in chromosomal complex package during cell division, and cytoplasmic survivin plays an important role in inhibiting apoptosis (32, 33). Immunofluorescence staining showed similar levels of survivin protein in K8+/+ and K8−/− nuclei, but survivin was particularly up-regulated in the cytoplasm of the K8−/− colonocytes (Fig. 4Bc and d), suggesting that survivin plays an important role in conferring resistance to apoptosis.
K8−/− Colonocytes Have Altered β4-Integrin Expression.
Studies in keratinocytes showed that cytoplasmic survivin can be down-regulated via blockade of β1-integrin, thereby leading to increased anoikis (34). This finding led us to compare integrin expression in K8−/− and K8+/+ colons. K8−/− colonocytes, particularly in the proximal colon [where inflammation is more prominent than in the distal colon (Fig. S3)], express higher levels of β4-integrin (Figs. 4C and 5A) but not β1-integrin (Fig. 5 B and C) compared with K8+/+ colons. β4-integrin is known to be distributed uniformly along the crypt–villus axis (35), and the increase in β4-integrin staining is seen in both the base and tip of the K8−/− colonic crypts (Fig. 4C).
Fig. 5.
K8−/− colons have altered β4- but not β1-integrin expression. (A) Lysates from proximal (PC) and distal colons (DC) shown from three independent age- and sex-matched pairs of K8+/+ and K8−/− mice were blotted with antibodies to β4-integrin and actin. Note the increase in β4-integrin (arrow) in proximal colons of K8−/− mice. (B) Lysates from proximal (PC) and distal colons (DC) from K8+/+ and K8−/− mice were blotted with antibodies to β1-integrin and actin. The blot from a representative pair of K8 mice is shown. (C) Frozen colon sections from K8+/+ (a) and K8−/− (b) mice were double stained for β1-integrin (red) and nuclei (blue). (Scale bar: 10 μm.) L, lumen.
In Vivo Anti–β4-Integrin Antibody Administration Leads to Further Increase in Survivin, FAK Phosphorylation, and Inhibition of Apoptosis.
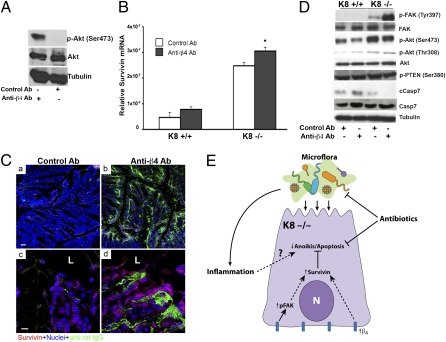
It is known that integrin-mediated cell-to-matrix contact leads to autophosphorylation of FAK and activation of downstream survival signals, probably through Akt (18). Based on this information, we examined whether the increased expression of β4-integrin in K8−/− colonocytes is functional by measuring consequent FAK phosphorylation in vivo using an activating anti–β4-integrin antibody. The activating nature of the anti–β4-integrin antibody we used was demonstrated by its ability to induce Akt phosphorylation using two mouse mammary cell lines, JC and EMT6 (results for JC are shown in Fig. 6A), as has been shown for other clones of anti–β4-integrin antibody using human breast and other cell lines (36, 37). We then tested the effect of β4-integrin antibody treatment on FAK phosphorylation and down-regulation of caspase activation in vivo. Notably, treatment of K8−/− mice with anti–β4-integrin antibody led to an increase in survivin mRNA (Fig. 6B) and protein (Fig. 6C).
Fig. 6.
In K8−/− colon, in vivo treatment with anti–β4-integrin antibody increases the expression of survivin and activation of FAK in concert with decreased apoptosis. (A) Lysates from the JC mouse mammary cell line treated with control or anti–β4-integrin antibody were analyzed by immunoblotting using antibodies to Akt, phospho-Akt, and tubulin. (B) RT-PCR analysis of survivin in colons of K8+/+ and K8−/− mice treated with either isotype control antibody (white bars) or anti–β4-integrin antibody (dark bars). Data are presented as mean relative survivin expression ± SEM (n = 3). *P < 0.05 when comparing control versus anti–β4-integrin antibody treatment. (C) Frozen colon sections from K8−/− mice treated with isotype control (a and c) or anti–β4-integrin antibody (b and d) were triple stained with anti-survivin (red), anti-rat IgG (green), and nuclei (blue). Higher-magnification images are shown in c and d. (Scale bars: 50 μm in a and b; 10 μm in c and d.) (D) Lysates from K8+/+ and K8−/− colons of mice treated with control antibody or anti–β4-integrin antibody were analyzed by blotting using antibodies to FAK, Akt, cleaved caspase-7 (cCasp7), caspase-7 (Casp7), tubulin, and phospho-FAK/Akt/PTEN. (E) Schematic model summarizing the proposed effect of K8 absence on colonocyte resistance to apoptosis through increased integrin signaling and increased cytoplasmic levels of antiapoptotic survivin. These effects, in the presence of the luminal microflora and/or inflammation, prevent apoptosis and probably contribute to the observed hyperproliferative epithelium in K8−/− colon.
The specificity of the anti–β4-integrin antibody is demonstrated by the fact that FITC anti-rat IgG stained β4-integrin in colonocytes of K8−/− mice treated with anti–β4-integrin antibody (Fig. 6Cb and d) but did not stain colonocytes from mice treated with the isotype control antibody (Fig. 6Ca and c). Furthermore, treatment with anti–β4-integrin antibody led to a robust increase in FAK pY397 in K8−/− colons (Fig. 6D). Although phosphorylation of Akt is increased in K8−/− compared with K8+/+ colons, immune blotting did not show significant differences in phospho-Akt between K8−/− mice treated with isotype control and K8−/− mice treated with anti–β4-integrin antibody (Fig. 6D). However, binding of β4-integrin leads not only to an increase in survivin expression (Fig. 6 B and C) but also to inhibition of apoptosis, as shown by the decrease in cleavage of caspase-7 (Fig. 6D). In addition, we assessed the activation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), because PTEN has been shown to play an important inhibitory role in cell survival and induction during apoptosis that is in part Akt dependent (38). We do not observe any evidence of PTEN activation (Fig. 6D). Collectively, these findings indicate that the up-regulation of β4-integrin in the K8−/− colon is likely to contribute to the observed inhibition of apoptosis via FAK activation.
Discussion
K8−/− and K18−/− (or keratin mutant) hepatocytes have similarly increased susceptibility to injury and apoptosis (5, 12, 13) because of the obligate heteropolymeric nature of K8/K18 and because, under basal conditions, K8 and K18 are the only keratins in adult hepatocytes (5). In the colon, K8 is the major type II keratin in mice (4) and humans (17) and is the major keratin that manifests a phenotype when absent (9, 10, 39) probably because of the functional redundancy of the type I keratins in the colon and the limited expression of K7 compared with K8 (5). Of the remaining keratins (K7, K18, K19, K20) concurrently expressed in the colon, the absence of K18 (14) or K19 (40, 41) or the overexpression of the dominant negative K20 (4) do not manifest an overt phenotype (to our knowledge, K7−/− mice have not been reported). In the liver, K8 is antiapoptotic; in the colon, as shown here, its absence confers resistance to apoptosis but only under normal physiological conditions that include the presence of resident microflora. The increased susceptibility of keratin-lacking or keratin-mutant hepatocytes to apoptosis, compared with their wild-type counterparts, is context dependent, in that differences are unmasked when apoptosis is induced by Fas but not by TNF (26, 42). Similar context-dependent bacteria-modulated effects also may occur in the colon, but this possibility remains to be investigated. It also is plausible that absence of normal keratins induces resistance to apoptosis as part of a normal stress response to luminal bacteria.
The mechanism by which microflora provide an antiapoptotic effect in the absence of colonocyte keratins is likely to be multifactorial. A relevant mechanism demonstrated herein is the enhanced integrin signaling in the K8−/− colon, which activates FAK and is mimicked and enhanced by administration of β4-integrin antibody. We also link K8 absence to increased levels of survivin, which is known to be involved, together with an activated FAK, in promoting cell survival (43). Other potential microflora effects that render keratin absence antiapoptotic may be related to quantitative or qualitative changes in the Toll-like receptors (TLRs), which are required for the intestinal homeostasis and protection of intestinal epithelial cells from injury (44, 45). For example, mistargeting or altered expression of apical or basolateral receptors in K8-null enterocytes, as seen in the small intestine (39), colon (9), and here for β4-integrin in the colon (Fig. 4), also may occur for one or more TLRs. A survey of the microarray results of several TLRs showed that TLR9 mRNA increased 2.5-fold, (particularly in the proximal colon; Fig. S4), a finding that was confirmed by immunohistochemistry and immunoblotting. Although the exact mechanism is not clear, there appears to be a link between the absence of K8 and up-regulation of β4-integrin expression in the colon. This notion is supported further by the down-regulation of β4-integrin in transgenic mice overexpressing human K8 (Fig. S5).
The importance of the microflora also is highlighted by the difference in resistance to apoptosis observed in the K8−/− colon and small intestine (jejunum). The human colon harbors 1013–1014 bacteria, whereas the proximal small intestine is estimated to have 103–104 bacteria (46). Factors other than microflora also may be involved in the differential resistance to apoptosis, because the small intestine and colon epithelium differ in many respects, ranging from morphologic to functional states. It is unlikely that differences in the composition of the keratin network in K8−/− colon and small intestine account for the difference in the two sites’ resistance to apoptosis, because previous studies showed similar alterations at the two sites in the remaining keratins (K7, K18, K19, and K20) (10, 39).
Colonic inflammation parallels the presence of luminal microflora. It is possible that the observed resistance of K8−/− colonocytes to apoptosis may be secondary to, or influenced by, inflammation. Inflammation is difficult to assess in the absence of luminal microflora because inflammation and microflora go hand in hand. Despite the different genetic or chemical mechanisms used to generate the colitis models, none of the current models develop colonic inflammation in a germ-free environment (47). Therefore we investigated and compared resistance to apoptosis in another immune-based model of chronic colitis, TCRα−/− mice. We did not observe such resistance to apoptosis in the TCRα−/− model of colitis, which by definition harbors an inflamed epithelium (31). However, our studies do not control for possible contributions arising from strain differences or differences in inflammation and microbial environment in the two colitis models. The findings herein highlight the importance of differences (such as microflora) in the microenvironments of hepatocytes and colonocytes; these differences lead to a paradoxical effect in the colon. If this effect also applies to hepatocytes, it might serve (e.g., through microflora-mimetic stimulation) to protect them from hepatotoxic injury. Taken together, these findings demonstrate that the absence of K8, coupled with the presence of microflora, renders colonocytes resistant to apoptosis. This resistance appears to be mediated, at least in part, by the overexpression of β4-integrin that can be linked to an increase in survivin and subsequent decreased activation of caspases (Fig. 6E).
The colonic hyperplasia that is observed in K8−/− mice (9, 10) probably results from both decreased apoptosis (Figs. 2 and 3 and Fig. S1) and increased proliferation (Fig. 4). We used two forms of stress to induce apoptosis: (i) colonocyte (colonic crypts) or whole-colon tissue isolation (organoids) followed by ex vivo culture, and (ii) application of known apoptosis-inducing agents ex vivo. The former method is similar to the classically described anoikis (a spontaneous form of apoptosis resulting from detachment from the cellular matrix), which occurs upon suppression of β4-integrin signaling (18). Therefore, the endogenous up-regulation of β4-integrin signaling in situ in K8−/− colon (Fig. 6) provides one likely mechanism for the observed protection from apoptosis. This protection occurs at the crypt surface, whereas the increase in proliferation occurs below this level, mostly involving the crypt from its base to ≈75% of its upward length (based on the Ki67 staining; Fig. 4A). These findings suggest that both resistance to apoptosis and the increase in proliferation probably account for the colonic hyperplasia. The microarray data and pathway analysis also indicated that the growth and differentiation pathway is the second most significantly altered pathway (Table S4), providing further indirect evidence for the dual, and probably regionally distinct, enhanced antiapoptosis and hyperproliferation signals as a consequence of the combined absence of K8 and presence of microflora. Other contributions to enhanced cell proliferation in the K8−/− colon probably are related to the mistargeting and alteration of the stability of membrane proteins such as transporters that can modulate intracellular pH and enhance cell proliferation (9).
Materials and Methods
Mice and in Vivo Experiments.
K8−/− mice (Jackson Laboratory); and their wild-type littermates, in an FVB/N background, were generated by interbreeding K8+/− mice as described (11). Age- and sex-matched mice (3–4 mo old) were studied. For antibiotic treatment, K8+/+ and K8−/− mice were given vancomycin and imipenem in their drinking water (50 mg/kg body weight/d for 8 wk) starting at postnatal d 18 or 19 (11). A group of K8+/+ and K8−/− mice received 200 μg anti–β4–integrin antibody (clone 346–11A) or control isotype antibody (rat IgG2a; BD Biosciences) i.p. every day for 3 d, and colons were harvested on d 4. In separate experiments, FVB/N mice were fasted overnight, and apoptosis was induced by i.p. injection of anti-Fas antibody (0.15 μg/g) (BD Biosciences) as described (42). After 4 h, the livers were harvested, and their homogenates were used as positive controls for caspase cleavage and apoptosis. TCRα+/+ and TCRα−/− mice on a C57BL/6 background were obtained from the Jackson Laboratory. Mice overexpressing human K8 were generated and maintained as described previously (48). All animals were treated according to National Institutes of Health guidelines and an approved animal institutional protocol.
Colonocyte Isolation and Intestine and Other Cell Culture.
Colonocytes were isolated as described (9). Isolated colonocytes were used for microarray analysis (see below) or were cultured in Dulbecco's modified Eagle medium supplemented with 4.5 g/L glucose, 25 mM Hepes, 5 ng/mL recombinant human epidermal growth factor, 0.2 IU/mL insulin, 5% FBS, 50 μg/mL penicillin, and 50 μg/mL streptomycin at 37 °C for 1 h. For organoid whole-colon or small intestine cultures, organs were opened longitudinally and were rinsed gently and quickly in incubation medium (RPMI-1640 plus 10% heat-inactivated FCS and antibiotics). The intestines then were cut into 5- to 7-mm pieces and incubated in the culture medium described above for 1 h at 37 °C, 5% CO2/95% O2. The incubations were carried out in the presence or absence of the apoptosis-inducing agents staurosporine (4 μM) (Cayman Chemical) and Fas antibody (500 ng/mL).
Activity of the anti–β4-integrin antibody or isotype control was tested in cultured mouse mammary cell lines (JC and EMT6; American Type Culture Collection). The cells were serum starved overnight, trypsinized from the dishes, and washed with PBS. The cells (5 × 106/mL) then were cultured at 37 °C for 6–8 h in the presence of anti–β4-integrin or isotype control antibody (6 μg/mL).
Real-Time PCR.
Total colon RNA was isolated using an RNeasy midi kit and converted into cDNA using a SuperScript II reverse transcriptase kit as recommended by the supplier (Invitrogen). qPCR was performed with an ABI Prism 7900 Sequence Detection System as described (49). Target genes (Table S3) were amplified using specific primers and SYBR Green PCR Master Mix. Gene expression levels were normalized to the housekeeping gene GAPDH. Bacterial DNA was extracted from freshly collected stools using the QIAamp DNA stool kit (Qiagen). Escherichia coli plasmid with a known amount of DNA (a gift from David Relman, Stanford University, Stanford, CA) served as a positive control, and stool DNA qPCR was performed as described (50).
Histology.
Freshly harvested colons or organ-culture colon pieces from K8+/+ and K8−/− mice were embedded in optimum cutting temperature (OCT) compound and frozen at –80 °C. Frozen 6-μm tissue sections were fixed in acetone and blocked with PBS containing 2% BSA and 2% goat serum (11, 51). The tissue sections were incubated with antibodies directed to cleaved caspase-7 (Cell Signaling Technology), K19 (Troma III; Developmental Studies Hybridoma Bank), survivin (Abcam), β4-integrin (BD Biosciences), or β1-integrin (R&D Systems) followed by washing and then incubation with the secondary antibodies, Texas Red or FITC goat anti-rabbit or anti-rat IgG (Jackson ImmunoResearch). Nuclei were stained after RNase treatment (51) using either Toto-3 or DAPI. Images were captured using a Zeiss LSM510 confocal microscope. Ki67 and PCNA staining of paraffin-embedded colon sections was performed by Histo-Tec Laboratory.
TUNEL Staining.
Organ-culture colon pieces from K8+/+ and K8−/− mice were embedded in OCT compound and frozen at –80 °C. Frozen tissue sections (6 μm) were stained using ApopTag Red In Situ Apoptosis Detection Kit (Millipore).
Immunoblotting.
Total liver and intestine homogenates were prepared in sample buffer containing 3% SDS. Except where indicated, we used total colons, because colons are shorter in K8−/− mice than in K8+/+ mice. Isolated colonocyte lysates were prepared in the same buffer by shearing with a 1-mL syringe mounted with a 21-G needle. Similarly, cell lysates were prepared from cultured JC and EMT6 cell lines by scraping the cells from the wells and shearing. Proteins were separated using SDS/PAGE and then were transferred to membranes. Membranes were blotted with the primary antibodies: anti-caspases, anti-cleaved caspases, anti-FAK, anti-pPTEN, anti–pAkt, anti-Akt (Cell Signaling Technology); anti-pFAK (Upstate Biotechnology); anti-K8 (Troma I); anti-K8 pS79 (LJ4) (51); anti–β4-integrin (BD Biosciences); anti–β1-integrin (R&D Systems); anti-tubulin, or anti-actin (NeoMarkers). Proteins were visualized using Western LightningTM Chemiluminescence Plus (Perkin-Elmer).
Statistical Analysis.
Data are expressed as means ± SEM from at least three independent experiments. Significance of differences was determined using the two-tailed Student's t test and ANOVA. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Robert Oshima and Helene Baribault for providing the K8−/− mice; Evelyn Resurreccion for tissue sectioning and fluorescence staining; Jean Chen, Alexander Hanganu, and Kris Morrow for assisting with qPCR, colon homogenization, and figure preparation, respectively; and Elisabeth Bik and Daniel DiGiulio for guidance on microbial DNA qPCR. This work was supported by National Institutes of Health Grants R01 DK47918 (to M.B.O.), K08 DK069385 (to A.H.), DK56339 (to Stanford University), and DK34933 (to the University of Michigan), a European Union FP7 International Reintegration Grant, and grants from the Academy of Finland and the Juselius Foundation (to D.M.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010833108/-/DCSupplemental.
References
- 1.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 2.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 3.Schweizer J, et al. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Q, et al. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol Biol Cell. 2003;14:2959–2971. doi: 10.1091/mbc.E03-02-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku NO, Gish R, Wright TL, Omary MB. Keratin 8 mutations in patients with cryptogenic liver disease. N Engl J Med. 2001;344:1580–1587. doi: 10.1056/NEJM200105243442103. [DOI] [PubMed] [Google Scholar]
- 7.Strnad P, et al. Keratin variants associate with progression of fibrosis during chronic hepatitis C infection. Hepatology. 2006;43:1354–1363. doi: 10.1002/hep.21211. [DOI] [PubMed] [Google Scholar]
- 8.Strnad P, et al. Acute Liver Failure Study Group Keratin variants predispose to acute liver failure and adverse outcome: Race and ethnic associations. Gastroenterology. 2010;139:828–835. doi: 10.1053/j.gastro.2010.06.007. 835, e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol. 2004;164:911–921. doi: 10.1083/jcb.200308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- 11.Habtezion A, Toivola DM, Butcher EC, Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci. 2005;118:1971–1980. doi: 10.1242/jcs.02316. [DOI] [PubMed] [Google Scholar]
- 12.Leifeld L, et al. Keratin 18 provides resistance to Fas-mediated liver failure in mice. Eur J Clin Invest. 2009;39:481–488. doi: 10.1111/j.1365-2362.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 13.Marceau N, et al. Dual roles of intermediate filaments in apoptosis. Exp Cell Res. 2007;313:2265–2281. doi: 10.1016/j.yexcr.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Magin TM, et al. Lessons from keratin 18 knockout mice: Formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J Cell Biol. 1998;140:1441–1451. doi: 10.1083/jcb.140.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesse M, et al. A mutation of keratin 18 within the coil 1A consensus motif causes widespread keratin aggregation but cell type-restricted lethality in mice. Exp Cell Res. 2007;313:3127–3140. doi: 10.1016/j.yexcr.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Owens DW, et al. Human keratin 8 mutations that disturb filament assembly observed in inflammatory bowel disease patients. J Cell Sci. 2004;117:1989–1999. doi: 10.1242/jcs.01043. [DOI] [PubMed] [Google Scholar]
- 17.Tao GZ, et al. Analysis of keratin polypeptides 8 and 19 variants in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:857–864. doi: 10.1016/j.cgh.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 19.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: More than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 22.Bullen TF, et al. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 23.Ku NO, et al. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest. 1996;98:1034–1046. doi: 10.1172/JCI118864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loranger A, et al. Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am J Pathol. 1997;151:1673–1683. [PMC free article] [PubMed] [Google Scholar]
- 25.Toivola DM, et al. Protein phosphatase inhibition in normal and keratin 8/18 assembly-incompetent mouse strains supports a functional role of keratin intermediate filaments in preserving hepatocyte integrity. Hepatology. 1998;28:116–128. doi: 10.1002/hep.510280117. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert S, Loranger A, Daigle N, Marceau N. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J Cell Biol. 2001;154:763–773. doi: 10.1083/jcb.200102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caulin C, Ware CF, Magin TM, Oshima RG. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000;149:17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zatloukal K, et al. Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am J Pathol. 2000;156:1263–1274. doi: 10.1016/S0002-9440(10)64997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao GZ, et al. Monitoring of epithelial cell caspase activation via detection of durable keratin fragment formation. J Pathol. 2008;215:164–174. doi: 10.1002/path.2344. [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P, et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 32.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: Molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 33.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009;8:2708–2710. doi: 10.4161/cc.8.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marconi A, et al. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells. 2007;25:149–155. doi: 10.1634/stemcells.2006-0165. [DOI] [PubMed] [Google Scholar]
- 35.Beaulieu JF. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- 36.Tang K, Nie D, Cai Y, Honn KV. The beta4 integrin subunit rescues A431 cells from apoptosis through a PI3K/Akt kinase signaling pathway. Biochem Biophys Res Commun. 1999;264:127–132. doi: 10.1006/bbrc.1999.1496. [DOI] [PubMed] [Google Scholar]
- 37.Gilcrease MZ, Zhou X, Welch K. Adhesion-independent alpha6beta4 integrin clustering is mediated by phosphatidylinositol 3-kinase. Cancer Res. 2004;64:7395–7398. doi: 10.1158/0008-5472.CAN-04-1809. [DOI] [PubMed] [Google Scholar]
- 38.Weng L, Brown J, Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum Mol Genet. 2001;10:237–242. doi: 10.1093/hmg/10.3.237. [DOI] [PubMed] [Google Scholar]
- 39.Ameen NA, Figueroa Y, Salas PJ. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J Cell Sci. 2001;114:563–575. doi: 10.1242/jcs.114.3.563. [DOI] [PubMed] [Google Scholar]
- 40.Tamai Y, et al. Cytokeratins 8 and 19 in the mouse placental development. J Cell Biol. 2000;151:563–572. doi: 10.1083/jcb.151.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ku NO, Soetikno RM, Omary MB. Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology. 2003;37:1006–1014. doi: 10.1053/jhep.2003.50181. [DOI] [PubMed] [Google Scholar]
- 43.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 44.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Gribar SC, Richardson WM, Sodhi CP, Hackam DJ. No longer an innocent bystander: Epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol Med. 2008;14:645–659. doi: 10.2119/2008-00035.Gribar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao WL, Lee YK. Microflora of the gastrointestinal tract: A review. Methods Mol Biol. 2004;268:491–502. doi: 10.1385/1-59259-766-1:491. [DOI] [PubMed] [Google Scholar]
- 47.Elson CO, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 48.Nakamichi I, et al. Keratin 8 overexpression promotes mouse Mallory body formation. J Cell Biol. 2005;171:931–937. doi: 10.1083/jcb.200507093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong B, et al. Organ-specific stress induces mouse pancreatic keratin overexpression in association with NF-kappaB activation. J Cell Sci. 2004;117:1709–1719. doi: 10.1242/jcs.01016. [DOI] [PubMed] [Google Scholar]
- 50.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ku NO, et al. Studying simple epithelial keratins in cells and tissues. Methods Cell Biol. 2004;78:489–517. doi: 10.1016/s0091-679x(04)78017-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.