Abstract
We report on a 70-year-old woman with partial complex status epilepticus who was initially diagnosed with herpes simplex-2 (HSV-2) encephalitis, based on brain magnetic resonance imaging (MRI) findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis and HSV-2 DNA detection by polymerase chain reaction (PCR) in the CSF, but without improvement on intravenous acyclovir. Anti-Ri antibodies were positive and computed tomography (CT) investigations revealed a small cell carcinoma at biopsy suggesting paraneoplastic encephalitis. The outcome was unfavourable and the autopsy showed typical features of paraneoplastic encephalitis but no evidence of viral inclusions. This case report is interesting because: (1) it is the first report of an autopsy proven paraneoplastic widespread encephalitis with anti-Ri antibodies; (2) despite a positive HSV-2 PCR in the CSF, there was no sign of herpetic infections of the nervous system; and (3) it illustrates the fact that if paraneoplastic antibodies are usually good markers of the underlying tumour, they are not always predictive of neurological deficits.
BACKGROUND
Paraneoplastic encephalitis which is usually limbic (PLE) was first described in 1960 as “subacute encephalitis of the later adult life mainly affecting the limbic areas”.1 Its classical clinical features include memory impairment or confusion associated to psychiatric symptoms and epileptic seizures. This clinical picture correlates with lesions classically involving mesio-temporal regions.2–4 In analogy with other paraneoplastic syndromes, PLE is likely caused by an immunological cross-reaction between antigens expressed by the tumour cells and autoantigens of the nervous system.2 Small cell lung carcinoma with anti-Hu antibodies (or anti-neuronal nucleus antibodies type 1, ANNA-1) is the most frequently associated neoplasia, but several others antibodies have been found.2,5–7
To the best of our knowledge, we report the first case of widespread paraneoplastic encephalitis confirmed by autopsy associated with anti-Ri antibodies (ANNA-2). The diagnosis was difficult because of a positive human herpes simplex type 2 (HSV-2) polymerase chain reaction (PCR) in the cerebrospinal fluid (CSF), but was confirmed at autopsy. We will review the immunological mechanisms of the disease.
CASE PRESENTATION
A 70-year-old woman was admitted to our department because of stupor. She was a heavy tobacco smoker (>100 UPY) and suffered 3 years earlier from urinary bladder cancer without local or distant metastases that was treated by cystectomy and ileal bladder confection. Five months before admission, her family noticed that she developed aggressiveness and strange behaviours. She also suffered, for the first time, from two generalised convulsive epileptic seizures but refused to see a doctor. While on vacation abroad, she developed progressive difficulties in verbal and written comprehension. She was admitted to an outside hospital after a new generalised epileptic seizure, which caused rib fractures. The brain computed tomographic (CT) scan revealed a left temporal hypodensity. Clonazepam was started (1 mg/day) and the patient was transferred to a regional hospital in Switzerland.
There, she underwent a lumbar puncture that revealed a lymphocytic pleocytosis (10 cells/μl), slightly increased proteins (50.1 mg/dl, range 15–46), and normal sugar; a PCR for HSV-2 came back positive (real time PCR, Qiagen/Artus HSV-1/2 LC PCR Kit, Medizinische Laboratorien, Niederwangen, Switzerland). Her brain MRI showed T2 hypersignal in lateral temporal regions predominating on the left. There was no metabolic or electrolytic disturbance in the blood work-up. Clonazepam was increased and intravenous acyclovir was started (30 mg/kg/day) for 3 weeks. During this treatment, the patient progressively worsened, becoming somnolent. She had several episodes of forced deviation of the head and eyes to the right. A treatment of phenytoin was added (450 mg/day) and the patient was transferred to our hospital with the suspicion of non-convulsive status epilepticus.
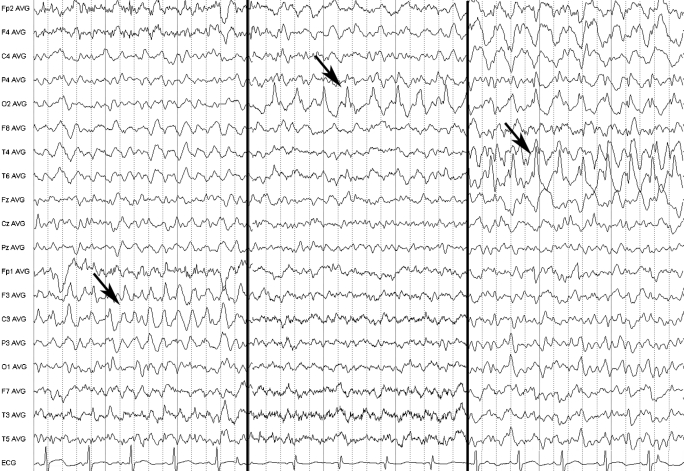
On admission, the patient was stuporous (Glasgow Coma Score (GCS) of 9), and displayed transient episodes of forced gaze and head version to the right and right peribuccal myoclonias. The electroencephalogram (EEG) confirmed a complex partial status epilepticus with waxing and waning multifocal bilateral epileptic activity (fig 1). Levetiracetam (2000 mg per day) and topiramate (200 mg per day) were prescribed as add-on medication.
Figure 1.
Three sequential electroencephalograms (30 mm/s; 70 μV/cm; HFF 70 Hz; LFF 0.5 Hz) in average referential montage showing left fronto-central (left panel), right occipital (middle panel) and right temporal (right panel) ongoing epileptic discharges (arrows).
INVESTIGATIONS
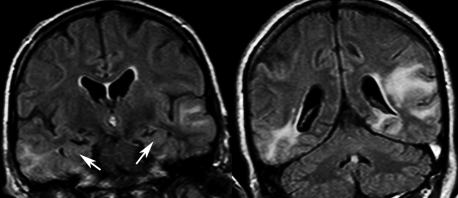
The work-up was repeated with a new lumbar puncture disclosing a slight lymphocytic pleocytosis (6 cells/μl), increased protein content (69.8 mg/dl) and normal sugar. There was an intrathecal IgG synthesis, whereas PCR for HSV type 1 and 2, human herpes type 6 (HHV6), varicella zoster virus (VZV), Epstein–Barr virus (EBV) and enteroviruses were negative. Blood serologies revealed signs of past infections of HSV, cytomegalovirus (CMV), EBV, and VZV, but no reactivation. General medical work-up including thyroid tests was unremarkable. Anti-nuclear antibodies were elevated (1/1280); however, antinucleoprotein, double stranded DNA, and anti-neutrophil cytoplasm antibodies were all negative. A second brain MRI confirmed the bilateral latero-temporal T2 hypersignals (fig 2) extending to the parietal region on the left, without gadolinium enhancement, diffusion or vascular abnormality. These abnormalities spared the mesial aspects of the temporal lobes.
Figure 2.
Fluid attenuated inverse recovery (FLAIR) magnetic resonance imaging showing bilateral temporo-lateral lesions sparing the medial aspect of the temporal lobes (arrows).
DIFFERENTIAL DIAGNOSIS
Herpes type 2 encephalitis versus paraneoplastic encephalitis
OUTCOME AND FOLLOW-UP
Because the partial complex status epilepticus was refractory to a combination of four medications, thiopental burst suppression therapy under EEG monitoring was started in the intensive care unit, together with steroid pulses (methylprednisolone, 125 mg/day) and increasing doses of leveraticetam (3000 mg/day) and topiramate (400 mg/day).
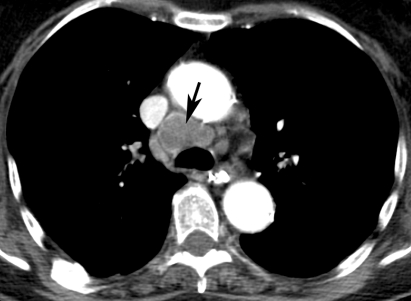
A thoraco-abdominal CT scan disclosed a pre-tracheal mass (fig 3); a biopsy during mediastinoscopy led to the diagnosis of small cell carcinoma. Four days after thiopental was withdrawn, the EEG again showed continuous multifocal ictal epileptiform activity.
Figure 3.
Contrast enhanced thoracic computed tomography scan showing 3 cm large pre-tracheal mass (arrow).
In this context, given the local infiltrative lung cancer, the refractory status epilepticus, and the poor overall patient’s conditions, a palliative attitude was decided; she died in a coma within a few days.
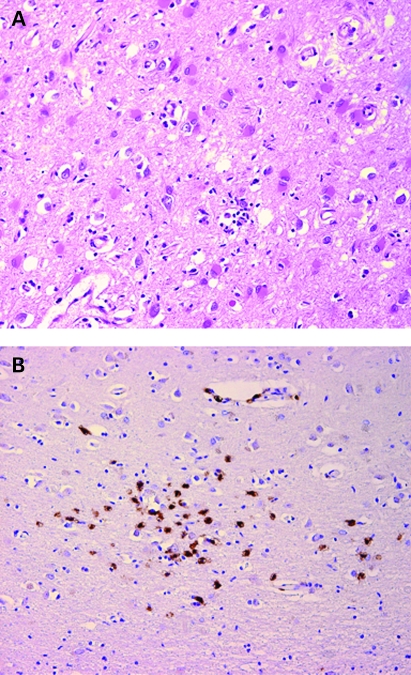
The assessment of blood autoantibodies (John Radcliffe Hospital, Oxford, UK) revealed positive anti-Ri (ANNA-2), whereas anti-Hu (ANNA-1), Anti Ma2 (Ta), anti-amphyphysin, and anti-CV2 (CRMP-5) results were negative. The autopsy confirmed the presence of lung small cell carcinoma, and showed widespread foci of encephalitis compatible with a paraneoplastic type of encephalitis. Cerebral lesions were predominant in the fronto-parieto-temporal cortex, insula, cingulate gyrus and brainstem, consisting of neuronal loss, reactive gliosis and multiple widespread lymphocytic infiltrates (fig 4a) of essentially CD8 T cells (fig 4b). There was no morphological (viral inclusions) or immunohistochemical evidence of HSV infection.
Figure 4.
(A) Histology of the cerebral cortex (cingulate gyrus) showing neuronal loss, reactive gliosis and lymphocytic infiltrate (haematoxylin and eosin 200×). (B) Immunohistochemistry showing that the lymphocytic infiltrates are composed mostly of CD8 T cells (CD8 100×).
DISCUSSION
Our patient displayed a rapidly progressive dementia over a 5 month course, complicated by a refractory complex partial status epilepticus. This exceptional case is very informative in several aspects.
First, our patient’s final diagnosis was widespread encephalitis due to lung small cell carcinoma with anti-Ri antibodies. These antibodies are directed against nuclear antigens of neurons widely distributed in the nervous system.8 They are classically described in opsoclonus-myoclonus associated with breast cancer.9 Less frequently, they have been associated with a large range of neurological syndromes such as brainstem symptoms,8,10 cerebellar ataxia, cranial or peripheral neuropathies. Supratentorial manifestations are rare,8 and status epilepticus has never been described as opposed to anti-Hu antibodies.11 To the best of our knowledge, only one case of cognitive and behavioural impairment attributed to encephalitis associated with anti-Ri antibodies was reported. However, the latter patient did not present with seizures, MRI abnormalities or an inflammatory CSF, and he remained clinically stable and no autopsy was performed.12 Our patient’s autopsy revealed multifocal widespread parcellar lymphocytic infiltration (mostly CD8+ T cells) of the brainstem and the cerebral cortex, associated with a strong gemistocitic gliotic reaction, features highly suggestive of paraneoplastic encephalitis.7,13 An apparently unusual feature of paraneoplastic encephalitis was the predominance of MRI hypersignals in the latero-temporal regions, but, in fact, the presence of lesions in extralimbic areas in “limbic encephalitis” appears to be the rule more than the exception.2 However, in our patient autopsy revealed diffuse brain inflammatory infiltration, largely exceeding the extent of lesions described in limbic encephalitis. Thus, in the presence of a suggestive clinical picture, it is important not to overlook the possibility of paraneoplastic encephalitis, even when facing atypical imaging or laboratory examinations. Indeed, in some series, neuroimaging shows atypical features in up to 35% of the cases.7
Usually paraneoplastic antibodies are specific of a neurological syndrome, such as anti-Yo and cerebellar degeneration or anti-NMDA and encephalitis,14,15 but sometimes paraneoplastic autoantibodies (typically such as anti-Ri) seem to be more specific of the underlying tumour than of the neurological syndrome.16 Indeed, in our case, anti-Hu (ANNA-1), anti-Ma2 (also called anti-Ta), anti-CV2 (CRMP-5), anti-voltage gated potassium channels (VGKC), anti-amphyphysin, or anti-voltage gated calcium channels (VGCC), which have all been associated with PLE, were not present in our patient.
The admitted hypothesis is that those paraneoplastic antibodies are directed against tumoral antigens and cross-react with antigens of the nervous system. However, if they are a good marker of a paraneoplastic immunological reaction, indicating that there is an underlying tumour, they may play a minor role in the effector phase of the inflammation against the nervous system. Indeed, it is thought that the cellular immunity is actually mostly involved in the pathological effects in this reaction.17 Thus, it has been recommended that when a paraneoplastic syndrome is suspected and the tumour is known, antibodies that have been attributed to this tumour should be looked for. Yet, there is an added degree of complexity since, for some antibodies, there can be several possible underlying tumours. This is the case for anti-RI antibodies. Indeed, in the largest published series, even if the aetiological tumours were lung cancer in 10/28 cases, the remaining 18 cases were due to a highly heterogeneous group of tumours.8 In any case, when facing a possible paraneoplastic syndrome, antibodies that have been associated with the given neurological syndrome should be looked for. However, when the neurological syndrome can be associated with many different antibodies, then an extensive battery of tests for paraneoplastic antibodies should be performed.16 In addition, sensitive techniques, such as positron emission tomography (PET) scan, should be employed to actively look for an underlying tumor.18
The initial diagnosis was HSV-2 encephalitis, as suggested by a positive PCR in the CSF, and the patient was treated accordingly. However, there are several elements speaking against this assumption: HSV-2 encephalitis is very rare in adults (<30 cases reported)19–22 and mostly occurs in immunocompromised patients; furthermore, the course is more benign than in HSV-1 encephalitis. Thus, the worsening condition of our patient over a period of several months, despite high doses of intravenous acyclovir, would be highly unusual. Finally, the autopsy showed no evidence of viral infection, as demonstrated by the absence of viral inclusions by optic microscopy and viral antigens by immunohistochemistry. This unfortunate case reminds us that the detection of viral genomic material by PCR in the CSF, while an invaluable tool for clinicians, may sometimes turn out to be irrelevant. HSV-2 DNA was likely amplified from the CSF of this patient, since the specificity of the PCR for HSV-2 can be as high as 99%.23 However, we hypothesise that this reactivation was an epiphenomenon, secondary to the inflammation related to the paraneoplastic syndrome, without a specific pathogenic role. Indeed, in this case the PCR reaction became positive after the 35th cycle, reflecting a weak viral load. Supporting this hypothesis is the fact that the second CSF PCR for HSV-2 was negative despite clinical worsening.
LEARNING POINTS
Despite its high sensitivity and specificity, a positive viral PCR does not rule out other aetiologies of encephalitis and caution should be applied in cases of atypical outcome.
Different neuroimaging aspects and several antibodies can been associated with paraneoplastic limbic encephalitis.
Paraneoplastic antibodies are markers of a paraneoplastic reaction; however, they sometimes do not predict the neurological clinical picture. So in absence of classically described antibodies, the search for paraneoplastic antibodies should be enlarged.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Brierley JB, Corsellis JAN, Hierons R, et al. Subacute encephalitis of the later adult age life mainly affecting the limbic areas. Brain 1960;83:357–68 [Google Scholar]
- 2.Anderson NE, Barber PA. Limbic encephalitis – a review. J Clin Neurosci 2008;15:961–71 [DOI] [PubMed] [Google Scholar]
- 3.Vernino S, Geschwind M, Boeve B. Autoimmune encephalopathies. Neurologist 2007;13:140–7 [DOI] [PubMed] [Google Scholar]
- 4.Alamowitch S, Graus F, Uchuya M, et al. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain 1997;120:923–8 [DOI] [PubMed] [Google Scholar]
- 5.Bataller L, Kleopa KA, Wu GF, et al. Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomes. J Neurol Neurosurg Psychiatry 2007;78:381–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson NE, Barber PA. Limbic encephalitis – a review. J Clin Neurosci 2008;15:961–71 [DOI] [PubMed] [Google Scholar]
- 7.Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000;123:1481–94 [DOI] [PubMed] [Google Scholar]
- 8.Pittock Sean J, Lucchinetti Claudia F, Lennon VA. Anti-neuronal nuclear autoantibody type 2: Paraneoplastic accompaniments. Ann Neurol 2003;53:580–7 [DOI] [PubMed] [Google Scholar]
- 9.Luque FA, Furneaux HM, Ferziger R, et al. Anti-Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann Neurol 1991;29:241–51 [DOI] [PubMed] [Google Scholar]
- 10.Fumal A, Jobe J, Pepin JL, et al. Intravenous immunoglobulins in paraneoplastic brainstem encephalitis with anti-Ri antibodies. J Neurol 2006;253:1360–1 [DOI] [PubMed] [Google Scholar]
- 11.Kile SJ, Kim JC, Seyal M. Complex partial status epilepticus in paraneoplastic limbic encephalitis. Clin EEG Neurosci 2007;38:172–4 [DOI] [PubMed] [Google Scholar]
- 12.Harloff A, Hummel S, Kleinschmidt M, et al. Anti-Ri antibodies and limbic encephalitis in a patient with carcinoid tumour of the lung. J Neurol 2005;252:1404–5 [DOI] [PubMed] [Google Scholar]
- 13.Scaravilli F, An SF, Groves M, et al. The neuropathology of paraneoplastic syndromes. Brain Pathol 1999;9:251–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittock Sean J, Kryzer Thomas J, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol 2004;56:715–9 [DOI] [PubMed] [Google Scholar]
- 17.Roberts WK, Darnell RB. Neuroimmunology of the paraneoplastic neurological degenerations. Curr Opin Immunol 2004;16:616–22 [DOI] [PubMed] [Google Scholar]
- 18.Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 2004;127:2331–8 [DOI] [PubMed] [Google Scholar]
- 19.Berger JR, Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch Neurol 2008;65:596–600 [DOI] [PubMed] [Google Scholar]
- 20.Chu K, Kang DW, Lee JJ, et al. Atypical brainstem encephalitis caused by herpes simplex virus 2. Arch Neurol 2002;59:460–3 [DOI] [PubMed] [Google Scholar]
- 21.Harrison NA, MacDonald BK, Scott G, et al. Atypical herpes type 2 encephalitis associated with normal MRI imaging. J Neurol Neurosurg Psychiatry 2003;74:974–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter MD, Manian FA, Kershaw MA, et al. Herpes simplex virus type 2 encephalitis in an elderly immunocompetent male. South Med J 2007;100:1143–6 [DOI] [PubMed] [Google Scholar]
- 23.Tebas P, Nease RF, Storch GA. Use of the polymerase chain reaction in the diagnosis of herpes simplex encephalitis: a decision analysis model. Am J Med 1998;105:287–95 [DOI] [PubMed] [Google Scholar]