Figure 6.
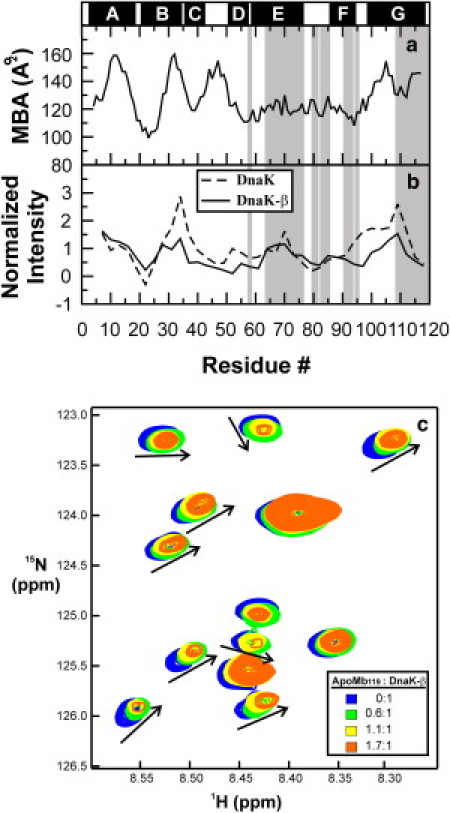
(a) Mean buried area (MBA) of the apoMb119 sequence calculated according to Rose et al. (71). High scores correspond to nonpolar regions of the chain. (b) Normalized experimental binding affinities of full-length DnaK (dashed line) and DnaK-β (solid line) for the apoMb119 sequence (72). (Shaded areas) Chain regions corresponding to the assigned backbone residues. (Solid bars above the plot) Position of the native α-helices in full-length apoMb. (c) 1H,15N-HSQC spectrum of 15N-DnaK-β chaperone in the absence and presence of variable amounts of unlabeled apoMb119, in 10 mM sodium acetate and 5% D2O, at pH 6.0. Spectra acquired at different stoichiometric ratios are color-coded according to the scheme provided in the legend. Specific resonance shifts observed upon addition of increasing amounts of apoMb119 (indicated by arrows to guide the eye).
