Figure 1.
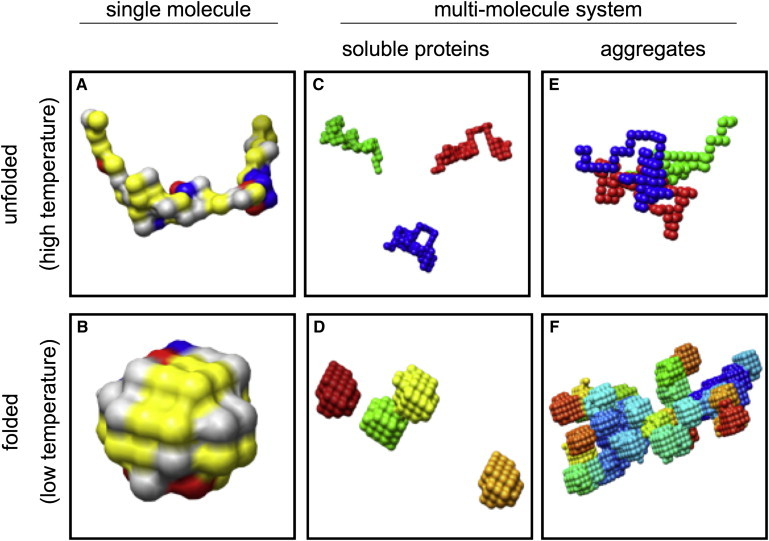
Schematic representation of aggregation behavior of lattice proteins. This figure illustrates a common problem with existing lattice protein models. Simulated as single molecules, proteins unfold at high temperatures (A), and fold into specific structures at low temperatures (B). In a multimolecule system, several scenarios for folding, unfolding, and aggregation are possible (C–F). As in nature, we would expect folded globular proteins to be soluble at low temperatures (D). However, existing models show a tendency to form large aggregates of folded proteins (F), due to (unphysically) strong attractions between hydrophilic residues. (See Fig. 4 for keys to the colors of panels A and B. In panels D–F, each protein chain has a different color.)
