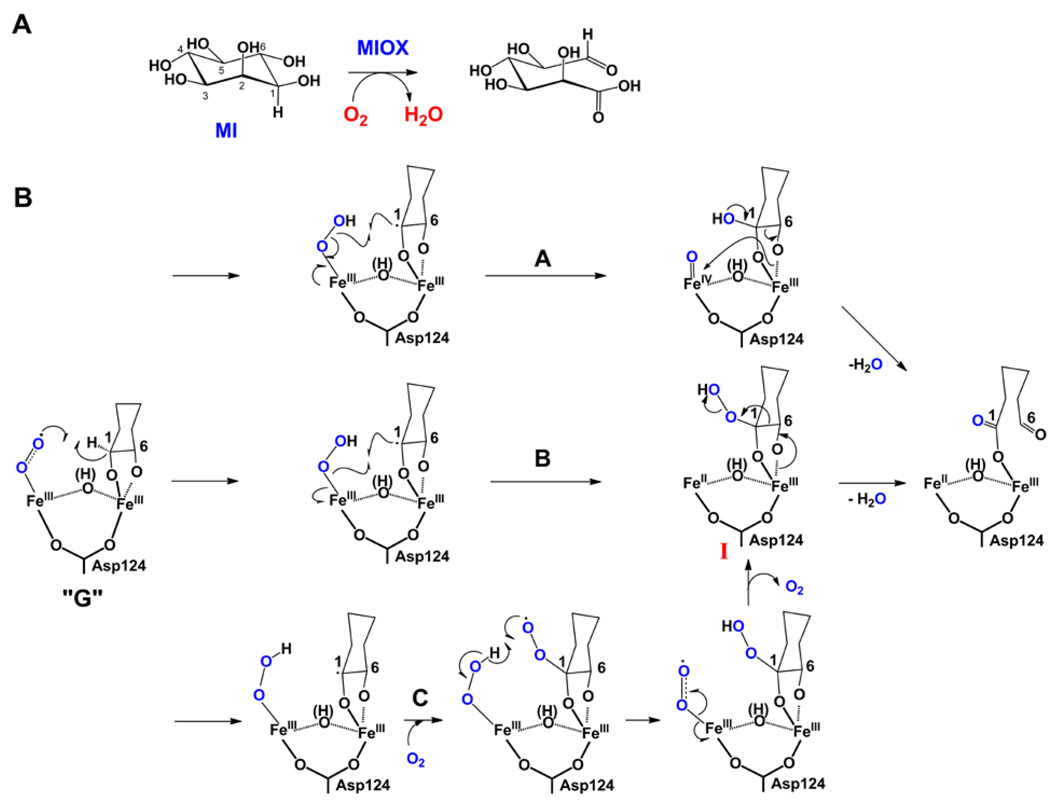
Figure 2.
(A) Reaction catalyzed by MIOX. B) Possible mechanisms for conversion of the superoxo-Fe2III/III complex, G, to the d-glucuronate product complex involving C1 hydroxylation coupled to peroxide O-O cleavage (pathway A), C1 hydroperoxylation by peroxyl-radical rebound (pathway B), or C1 hydroperoxylation by addition of a second molecule of O2 to the C1 radical (pathway C). In pathway A, C-C bond cleavage with the electrons of the incipient anion directly attacking the ferryl complex is also feasible, but this would lead to incorporation of the ferryl oxygen into the resulting hydrated aldehyde. Oxygen labeling experiments did not detect any oxygen derived from O2 in the aldehyde [45], requiring that for this mechanism to hold, either the oxygen of the ferryl intermediate underwent complete exchange, or the conversion of the initial hydrated product to the aldehyde occurred stereospecific and enzyme catalyzed.