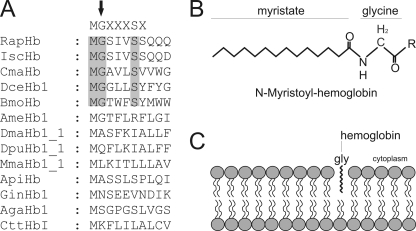
FIGURE 8.
Membrane-association of globins promoted by N-myristoylation. Identification of N-myristoylation signal sequences within the arthropod globins by computer predictions. N-Myristoylation sites are shaded gray. Consensus sequence for N-myristoylation is superimposed in the upper row. The N-terminal glycine required for the attachment of the fatty acid myristate is marked by an arrow. RapHb, R. appendiculatus hemoglobin; IscHb, I. scapularis hemoglobin; DceHb1, D. cervinus hemoglobin; BmoHb, B. mori hemoglobin; AmeHb1, A. mellifera hemoglobin; DmaHb1_1, D. magna hemoglobin domain 1; DpuHb1, Daphnia pulex hemoglobin domain 1; MmaHb1_1, M. macrocopa hemoglobin domain 1; ApiHb, Acyrthosiphon pisum hemoglobin; GinHb1, Gasterophilus intestinalis hemoglobin; AgaHb1, A. gambiae hemoglobin; CttHbI, Chironomous thummi thummi hemoglobin. A, myristoylated hemoglobin. After removal of the initiator Met residue, the 14-carbon saturated fatty acid myristate is covalently attached to the N-terminal glycine of eukaryotic proteins (B). Shown is the membrane association of globins by N-myristoylation (C).