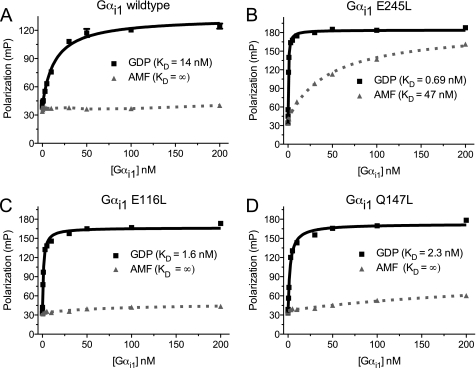
FIGURE 1.
WT Gαi1 and three affinity-enhanced mutants bind to the RGS14 GoLoco motif in a nucleotide-dependent manner. Binding isotherms were generated using FP measurements by increasing the concentration of Gαi1 protein while maintaining the concentration of FITC-labeled RGS14 GoLoco motif peptide constant at 350 pm. All experiments were performed in triplicate. The error bars represent S.E. A, Gαi1WT·GDP binds the RGS14 GoLoco motif with a dissociation constant (KD) of 14 nm (95% CI, 11–17 nm). No appreciable binding was observed when the Gαi1 protein was in its transition-state mimetic form, created with AMF. B, Gαi1E245L binds the RGS14 GoLoco motif with KD values of 0.69 nm (95% CI, 0.61–0.77 nm) and 47 nm (95% CI, 37–56 nm) in its GDP and GDP·AMF forms, respectively. C, Gαi1E116L·GDP binds the RGS14 GoLoco motif with a KD of 1.6 nm (95% CI, 1.4–1.7 nm), whereas no appreciable binding is observed with the AMF-activated Gαi1E116L. D, Gαi1Q147L·GDP binds the RGS14 GoLoco motif with a KD of 2.3 nm (95% CI, 1.9–2.6 nm), whereas no appreciable binding is observed in the AMF-activated form. mP, milli-Polarization units.