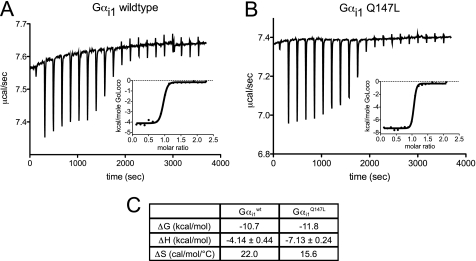
FIGURE 5.
Thermodynamic contributions to RGS14 GoLoco motif binding by WT and Gαi1Q147L subunits as determined by ITC. 30 μm solutions of each indicated Gαi1 subunit were separately titrated with repeated additions of 350 μm RGS14 GoLoco motif peptide. Calorimetric traces of heat released and enthalpic binding curves (insets) fitted with a one-site binding model are shown for Gαi1WT (A) and Gαi1Q147L (B). The stoichiometry of binding was ∼1:1 in all experiments (three independent titrations were performed for each protein). C, average values and 95% confidence intervals for enthalpy of binding (ΔH) are given for the triplicate titration experiments (p = 0.006). Gibbs free energy (ΔG) is calculated from FP binding affinity measurements, and entropy of binding is derived by ΔS = (ΔH − ΔG)/T.