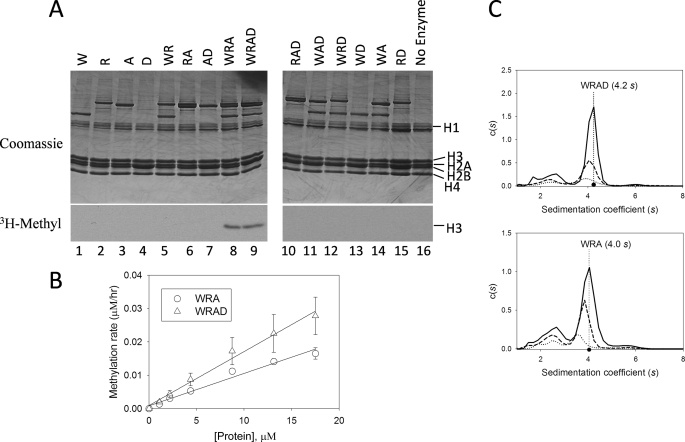
FIGURE 2.
WDR5, RbBP5, and Ash2L are required for the H3K4 methylation activity of WRAD. A, comparison of enzymatic activity of individual WRAD components and all possible binary, ternary, and quaternary complexes. W, WDR5; R, RbBP5; A, Ash2L; D, DPY-30. Histone methyltransferase assays were conducted for a period of 8 h using [3H]AdoMet and chicken core histones as the substrate. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panels) and fluorography (lower panels). A no-enzyme control is shown in lane 16. B, WRAD and WRA histone methyltransferase activity as a function of enzyme concentration. Methyltransferase activity assays were conducted with 25 μm [3H]AdoMet and 500 μm histone H3 peptide (residues 1–20) with varying concentrations of WRAD (open triangles) or WRA (open circles). Each point corresponds to the average of duplicate measurements with the error bars indicating the standard error of measurement. Linear regression fitting of the data gave slopes of 0.0016 and 0.001, and R2 values of 0.99 and 0.97 for WRAD and WRA complexes, respectively. C, diffusion-free sedimentation coefficient distributions (c(s)) derived from sedimentation velocity analytical ultracentrifugation of WRAD (upper panel) and WRA (lower panel) complexes at concentrations of 2.2 μm (solid line), 1.1 μm (dashed line), and 0.55 μm (dotted line).