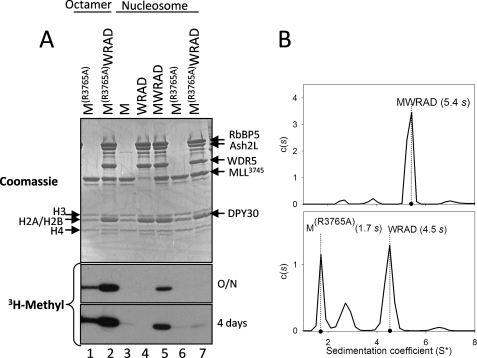
FIGURE 6.
Arginine 3765 of MLL1 is required for the interaction between MLL1 and WRAD and for nucleosomal histone H3 methylation. A, comparison of the enzymatic activity of wild-type (M) and R3765A (MR3765A) MLL1 SET domains in the presence and absence of WRAD using dialyzed histone octamers or reconstituted nucleosomes as substrates. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panel) and fluorography (3H-Methyl (lower panels), overnight (O/N) and 4-day exposures)). Lanes 1 and 2 are assays conducted with dialyzed histone octamers; lanes 3–7 are assays conducted with a reconstituted nucleosome substrate. B, diffusion-free sedimentation coefficient distribution (c(s)) derived from sedimentation velocity analytical ultracentrifugation of the MLL1 core complex assembled with wild type (upper panel) or the R3765A variant of MLL1 (lower panel). The experimental sedimentation coefficients (s) are indicated.