FIGURE 5.
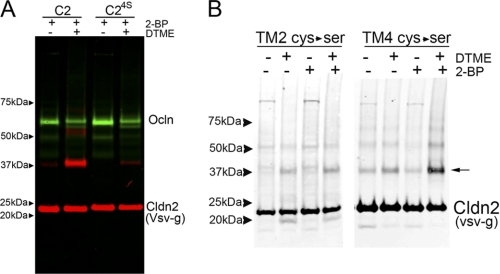
Cysteine cross-linking suggests proximity of the second transmembrane domains. A, cldn2 can be cross-linked with disulfide cross-linkers by perimembrane cysteines. MDCK cells induced to express wild-type cldn2 (C2) and cldn2 with perimembrane cysteines mutated to serines (C24S) were incubated overnight in 2-bromopalmitate to inhibit palmitoylation and then incubated without (−) and with (+) the cysteine cross-linker DTME. Cell extracts were subjected to SDS-PAGE and immunoblotted for VSV-g cldn2 (red) and occludin (green); wild-type cldn2 was cross-linked with much greater efficiency than cldn24S; cldn24S migrates as a dimer in BN-PAGE (data not shown). B, mutational analysis suggests that the second transmembrane domains may be in close proximity. MDCK cells stably transfected and induced to express VSV-g-tagged cldn2 with perimembrane cysteines mutated to serines after the second (TM2 Cys → Ser) or fourth (TM4 Cys → Ser) were incubated overnight without (−) or with (+) 2-bromopalmitate and then treated (+) with cysteine cross-linker. The only sample where cross-linking to dimer (arrow) was clearly dependent on preincubation with 2-bromopalmitate and DTME was TM4 (Cys → Ser), suggesting a critical role for perimembrane cysteines following the second transmembrane domain.