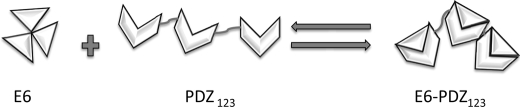
FIGURE 4.
Scheme for the interaction between HPV E6 and the PDZ domains of SAP97. E6 forms oligomers, which dissociate upon binding to SAP97 (k = ∼20–50 s−1). All three PDZ domain of SAP97 may bind one E6 molecule each, and a conformational change of the quaternary complex gives a similar hydrodynamic radius to that of apo PDZ123. Dissociation rates govern the affinities for HPV16 and HPV18 E6 proteins for the PDZ domains of SAP97. Residues outside the canonical binding site of the second PDZ domain are affected by the E6 protein in the binding reaction.