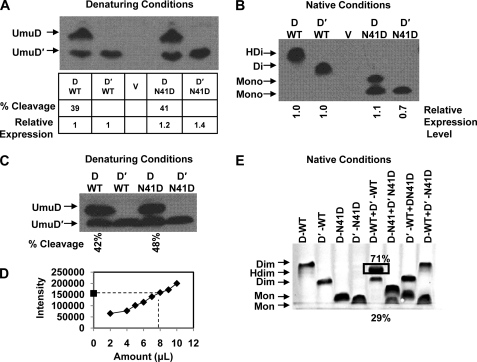
FIGURE 5.
UmuD expression levels, cleavage products, and heterodimer formation. Protein expression was induced by exposing cultures to 25 J/m2 UV light. A, an immunoblot of gel run under denaturing conditions shows the steady-state expression levels of UmuD from plasmids pGY9739 and pGY9738 in GW8017. Relative UmuD expression levels are shown below the blot, where UmuD N41D is normalized to wild-type UmuD, and UmuD′ N41D is normalized to wild-type UmuD′. Percent cleavage for UmuD2 and UmuD N41D is also indicated. B, an immunoblot of a gel under native conditions shows that UmuD2 and UmuD′2 are expressed as dimers, whereas UmuD N41D and UmuD′ N41D are expressed as monomers in vivo. There is no cleavage product seen under these conditions for wild-type UmuD2; however, the cleavage product for UmuD N41D is evident. C, shown is an immunoblot of identical samples from B under denaturing conditions. In this blot, both full-length and cleaved products are present in almost equivalent amounts, suggesting that a stable wild-type UmuDD′ heterodimer was observed in B. D, Western blots in A, B, and C were carried out to give a signal within the linear range of the assay. The intensity of the signal versus the amount of crude lysate loaded is shown. The point on the y axis of the graph represents the band from gels in A–C with highest intensity, which is the heterodimer in B. E, heterodimer formation is shown under equilibrium conditions using purified UmuD proteins in vitro. Native gel shows preferential heterodimer formation (box) when wild-type UmuD and UmuD′ proteins are combined in equivalent amounts. UmuD N41D and UmuD′ N41D do not form heterodimers as expected. Combining N41D variant and wild-type UmuD or UmuD′ also does not result in heterodimerization.