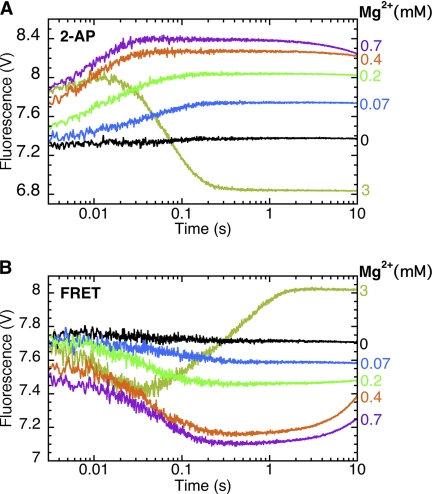
FIGURE 7.
A single metal ion is sufficient for open and closed ternary complex formation; the second metal ion is needed for nucleotide incorporation. A, stopped-flow fluorescence assay for step 2.1. The binary complex of Pol I(KF) and L:T(+1)2-AP:3′OH (Fig. 3B) was mixed with 2 mm dGTP. B, stopped-flow fluorescence assay for fingers-closing (step 2.2). The binary complex of 744-AEDANS Pol I(KF) and H:T(−8)D:3′OH DNA duplex (Fig. 3C) was mixed with 2 mm dTTP. In both experiments, MgCl2 was omitted from the Pol-DNA drive syringe and was present only in the syringe containing the complementary nucleotide. The reaction buffer in both syringes contained 1 mm EDTA, which served to limit the free Mg2+ concentration. As described by Bakhtina et al. (12), the total MgCl2 concentrations in the reaction were 0.07, 0.2, 0.4, and 0.7 mm (indicated in the figure), corresponding to free Mg2+ concentrations of 0.25, 0.81, 2, and 5.8 μm, and Mg-dNTP concentrations of 3.1, 9.8, 25, and 67 μm. Control reactions in the presence of 3 mm MgCl2 indicate the signal obtained when covalent dNTP incorporation takes place. The fluorescence changes observed between 1 and 10 s in the presence of 0.7 mm Mg2+ correspond to the slow addition of dNTP; these changes were absent in an otherwise identical reaction using the corresponding dideoxy-terminated DNA substrate.