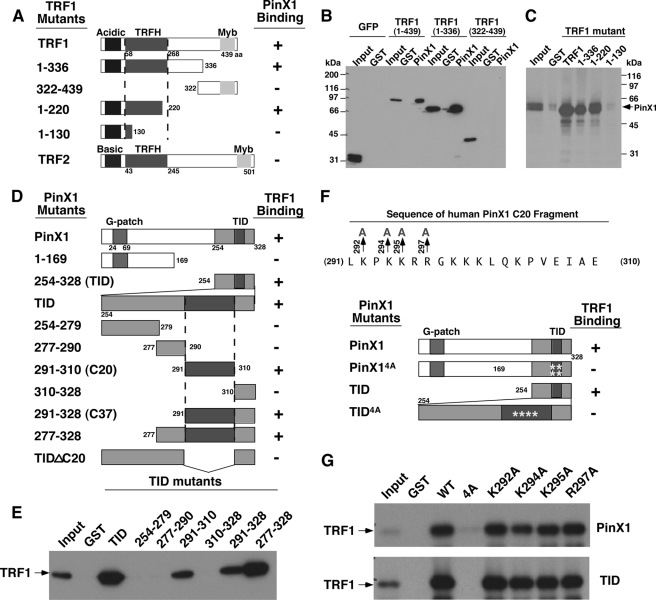
FIGURE 1.
The identification of the minimal domains responsible for the PinX1 and TRF1 interaction. A–C, PinX1 binds to the TRFH domain of TRF1. A series of TRF1 truncation mutants (A) were expressed in cells as GFP fusion proteins followed by GST pulldown with GST-PinX1 or control GST (B) or expressed in bacteria and purified as GST fusion proteins followed by GST pulldown with in vitro synthesized 35S-PinX1 (C). PinX1 binding of TRF1 mutants or TRF2 was summarized in A. D and E, a 20-amino acid domain (C20) in PinX1 is necessary and sufficient for binding to TRF1. A series of PinX1 truncation mutants (D) was expressed in bacteria and purified as GST fusion proteins followed by GST pulldown with in vitro synthesized 35S-TRF1 (E). TRF1 binding of PinX1 mutants was summarized in D. F and G, the mutation of multiple positively charged residues in C20 is required to abolish the PinX1-TRF1 interaction. The C20 fragment of PinX1 contains several positively charged residues, including Lys-292, Lys-294, Lys-295, and Arg-297 (F). Full-length PinX1 or its TID containing Ala substitutions of these residues individually or altogether were expressed in bacteria and purified as GST fusion proteins followed by GST pulldown with in vitro synthesized 35S-TRF1 (G). Note that the mutation of these charged residues individually in full-length PinX1 or its TID was not sufficient to disrupt the PinX1-TRF1 interaction.