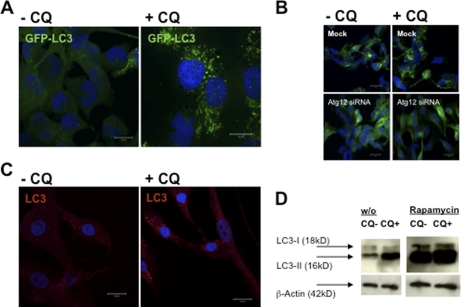
FIGURE 1.
Macroautophagy is a constitutively active process in human skeletal muscle cells. A, human rhabdomyosarcoma cells (CLL136) were stably transfected with a GFP-LC3 reporter construct and analyzed for GFP-LC3 turnover with and without lysosomal proteolysis blockade due to CQ treatment, 50 μm for 10 h. GFP-LC3 strongly accumulated in ring-shaped and cup-shaped cytosolic vesicles after 10 h of CQ treatment (A, right panel), suggesting that large numbers of GFP-LC3-labeled autophagosomes had formed and fused with lysosomes/late endosomes during the 10-h observation period. Cells were fixed, stained with DAPI nucleic acid stain, and analyzed by confocal microscopy. Scale bars, 20 μm. B, accumulation of GFP-LC3+ vesicles upon CQ treatment was dependent on macroautophagy, because siRNA-mediated silencing of atg12 abrogated accumulation of these vesicles. C, similar to the skeletal muscle cell line, human primary myoblasts stained with a monoclonal antibody specific for LC3 showed a detectable level of constitutive macroautophagy and a marked accumulation of LC3+ vesicular compartments upon CQ treatment. D, autophagosome-associated LC3 (called LC3-II) and free cytosolic LC3 (called LC3-I) can be distinguished by their apparent molecular weights in SDS-PAGE (16 and 18 kDa, respectively), and thus can be quantified separately in anti-LC3 immunoblots. Autophagosome-associated LC3-II strongly accumulated upon CQ treatment, further demonstrating that LC3-II-labeled autophagosomes were constitutively degraded in endosomes and/or lysosomes over the course of 10 h. In addition, inhibition of the mammalian target of rapamycin by rapamycin resulted in a substantial increase in LC3-II expression in both CQ-treated and untreated cells. w/o, without.