Abstract
Despite being a common cancer worldwide, management of transitional cell carcinoma of the bladder currently relies primarily on clinical staging and histopathologic parameters. Assaying alterations in molecular pathways can contribute valuable information that can accurately predict outcome and chemotherapeutic response in individual patients with bladder cancer. Medium- to high-throughput gene-expression profiling technologies are now allowing multiplexed assessment of alterations responsible for the genesis and progression of bladder tumors. These investigations employ global or pathway-based approaches to define molecular signatures that can predict prognosis independent of traditional clinical performance metrics. Prognostic panels generated using these strategies can also elucidate the biology of tumor progression and identify potential therapeutic targets.
Keywords: gene-expression profiling, global approach, microarray, multimarker analysis, pathway-specific approach, urothelial carcinoma
Bladder cancer is the fourth most common cancer in men in the USA [1]. In 2010, bladder cancer will account for an estimated 70,530 new cases and 14,680 deaths in the USA alone.
The most common presenting pathology of bladder tumors is transitional cell carcinoma (90% of cases), while squamous cell carcinomas, adenocarcinomas and other rare subtypes comprise a minority of cases [2]. Clinical staging and histopathologic parameters are currently the most commonly used prognostic tools. Of these, the Tumor, Node, Metastasis (TNM) staging system is one of the most widely used prognostic criteria clinically [3]. The final stage can only be determined in a cystectomy specimen, while other clinical staging procedures (during transurethral tumor resection, radiology, ultra-sonography, urine cytology, urine molecular testing, bimanual examination, and so on) may have a high rate of understaging, which might defer treatment and can worsen the final outcome [4,5]. Interpretation of histopathologic criteria, which represents an important component of patient staging, can also be hampered by significant variability among pathologists despite well-defined criteria for the diagnosis of urothelial carcinoma. Interobserver variability has also been identified in distinguishing between stage Ta versus T1 bladder tumors, and in grading these tumors [6,7]. This highlights the necessity to introduce more objective criteria in staging bladder cancer that can also act as robust indicators of prognosis.
It has now been clearly shown that alterations in molecular pathways that control cellular homeostasis may result in the formation of bladder cancer [8]. Several molecules and pathways have been identified and linked to this tumorigenic process. While major efforts to characterize molecular and pathway alterations have been undertaken to improve disease prognostication, only a few biomarkers of potential clinical relevance have been identified thus far. A major impediment to this discovery process has been the technological limitations that have restricted in-depth analyses of molecular aberrations that often occur in tandem, thereby deregulating normal cellular processes and resulting in the malignant phenotype. Traditional approaches have focused on profiling one molecule or a single pathway in an effort to better predict the clinical course of bladder cancer patients. However, researchers over the last decade have started investigating panels of markers that may lead to a better understanding of the tumor course of an individual patient.
The most well-characterized cellular process that is dysregulated in bladder tumorigenesis is cell cycle regulation. The cell cycle is primarily controlled by the p53 and retinoblastoma (Rb) pathways [9]. Besides the cell cycle, p53 is also involved in other processes related to tumorigenesis including angiogenesis, apoptosis and DNA repair [10]. Medium- to high-throughput profiling technologies are now enabling investigators to explore bladder cancer-related bio-markers across the entire genome and proteome. This has resulted in expansion of the potential for discovery of novel molecules and pathways that can not only serve as prognostic indicators but also as potential therapeutic targets in bladder cancer [11].
This article will initially outline the major well-characterized molecules and pathways associated with bladder cancer progression. It will then discuss the global and pathway-specific approaches towards gene-expression profiling, with a specific emphasis on identification of molecular signatures for bladder cancer outcome prediction.
Individual molecular alterations in bladder cancer
The identification of different molecules and pathways that can potentially predict outcomes in bladder cancer patients has also contributed to the understanding of the biologic mechanisms related to bladder tumorigenesis, recurrence and progression. The prognostic importance of the major pathways altered during these processes is described later.
Cell cycle regulation alterations
Two pathways primarily control the cell cycle: the p53 and Rb pathways. The p53 protein, encoded by the TP53 tumor-suppressor gene, inhibits cell cycle progression at the G1–S transition. TP53 mutations are common in invasive bladder cancer [12]. It has been reported that increased p53 nuclear immunoreactivity, which is potentially a surrogate indicator of TP53 mutation, is predictive of outcome particularly in patients with invasive organ-confined and lymph node-negative disease [13–15]. However, recent evidence from our group suggests that there may not be a perfect concordance between TP53 mutation and increased p53 immunoreactivity; the combined assessment of alterations at the gene and protein level in such scenarios can provide a more accurate assessment of prognosis than either marker alone [12].
p53 induces the transcription of p21WAF1/CIP1, which encodes for p21, a CDK inhibitor (CDKI) [16]. In bladder cancer patients, loss of p21 expression has been demonstrated to be an independent predictor of disease progression [17]. The Mdm2 protein controls p53 activity through an autoregulatory feedback loop [18]. Mdm2 degrades p53 following upregulation of the MDM2 promoter by elevated p53 levels. MDM2 amplification has been documented in bladder cancer, and has been shown to be more frequent in high-stage and high-grade tumors [19]. A single-nucleotide polymorphism in the MDM2 promoter, SNP309, is a frequent event in bladder cancer and predicts an earlier onset of non-muscle-invasive disease, and can be especially prognostic when linked to TP53 mutation status [20].
The transcription of MDM2 is inhibited by p14. The gene encoding for p14, p14ARF, is induced by E2F, which links the p53 pathway to the Rb pathway [8]. The RB gene encodes for the Rb protein, which plays a critical role in cell cycle regulation [21]. The active dephosphorylated Rb binds to and inhibits E2F [22]. In its phosphorylated state, Rb releases E2F that in turn is able to induce gene transcription for DNA synthesis. Mutations in the RB gene that inactivate Rb have been found in bladder cancer [23]. However, patients with tumors expressing elevated Rb levels as determined by immunohistochemistry have comparably poor outcomes to patients with tumors that have inactivating RB mutations [24]. This is postulated to occur through Rb hyperphosphorylation owing to loss of expression of the CDKI p16 and/or cyclin D1 overexpression [25].
Alterations in apoptosis
Apoptosis may be affected through two different mechanisms: the extrinsic and intrinsic pathways. The extrinsic pathway is activated by specific receptors on the cell membrane; the intrinsic pathway is activated through the mitochondria. Both pathways lead to activation of effector caspases, which induce the cellular changes leading to cell death. Receptors of the extrinsic pathway, such as Fas, belong to the TNF superfamily and have been shown to be bypassed during malignant transformation of bladder cancer cells [26]. Decreased Fas expression has been linked to tumor progression in transitional cell carcinoma [27].
The antiapoptotic protein Bcl-2 plays a major role in the intrinsic pathway. It controls the mitochondrial membrane permeability and inhibits caspase activation [10]. Increased Bcl-2 protein production is associated with decreased tumor-specific survival in T1G3 bladder carcinomas [28]. When combined with p53, Bcl-2 has been shown to be a good prognostic tool in predicting outcomes in patients with non-muscle-invasive bladder cancer [29].
Signal transduction alterations
Alterations in diverse pathways that transduce signals from cell surface receptors to the nucleus for transcription may result in the dysregulation of cellular homeostasis. In the case of bladder cancer, the Ras–MAPK and JAK–signal transducer and activator of transcription (STAT) signaling cascades are important signal transduction pathways.
Mutations in the FGF receptor 3 (FGFR3) gene that activate the Ras–MAPK pathway are common in non-invasive, low-grade papillary urothelial carcinomas [30]. Although Harvey rat sarcoma viral oncogene homolog gene (HRAS) mutations have been identified in urine cytologic preparations from low-grade bladder tumors [31], Ras mutations are not as common in Ta tumors. Our group has recently shown that HRAS is more likely to be overexpressed in Ta tumors that are not likely to progress, compared with those Ta tumors that have a higher risk of eventually progressing [32]. VEGF receptor 2 (VEGFR2; KDR/Flk-1), which mediates cellular responses of VEGF through the MAPK signaling cascade, is also overexpressed with increasing disease stage and muscle invasion [33]. Furthermore, VEGFR2 expression is predictive for nodal metastasis in bladder cancer [34], and also predicts progression of Ta tumors at first presentation [32].
The JAK family includes a group of tyrosine kinases that are activated by different cytokines and growth factors and influence various signaling pathways. The activation of JAK leads to the activation of STATs, which control the transcription of several genes including the one encoding for Bcl-2 [35]. In combination with other markers, STAT3 overexpression can predict increased risk of recurrence and decreased survival for patients with bladder cancer [36].
c-Fos and c-Jun function as transactivators downstream of several signal transduction cascades to control the transcription of genes involved in differentiation, apoptosis and proliferation. While increased Jun expression has been associated with increasing tumor stage [37] and poor recurrence-free and overall survival rates in bladder cancer patients [36], patients with c-Fos expression are more likely to have a higher tumor grade [38].
Alterations in angiogenesis
Several factors contribute to the growth of blood vessels into a tumor, which is responsible for providing oxygen and nutrients and promoting malignant progression. Microvessel density, a surrogate marker for angiogenesis [39], has been significantly associated with bladder cancer outcomes [40]. Besides microvessel density, hypoxia-inducible factors (HIF-1α and HIF-2α) are important angiogenic molecules as they control a cell’s response to a hypoxic stress [41]. Overexpression of HIF-1α has been demonstrated in bladder cancer patients with poor prognosis; its prognostic value is even more significant when combined with p53 [42,43]. HIF-1α has also been shown to predict recurrence and survival in non-muscle-invasive tumors [44].
The most crucial molecule in angiogenesis, VEGF, which is induced by HIF, is highly expressed in patients with non-muscle-invasive bladder cancer who recur early and show a high rate of progression to an invasive phenotype [45]. Our group recently showed that high VEGF levels in combination with high VEGFR2 and HRAS expression can predict progression of Ta tumors at first presentation [32]. Furthermore, metastatic patients and patients with poor outcome have significantly higher serum VEGF levels than those with localized disease [46].
p53 has also been shown to have an impact on tumor angiogenesis. p53 upregulates thrombospondin-1, which functions as an antiangiogenic factor. Thrombospondin-1 expression has been strongly correlated to disease recurrence and overall survival [47].
Combination of molecular alterations
Broader characterization of individually prognostic markers in bladder cancer has paved the way toward assessing multiple markers in tandem at the protein level in an effort to increase their prognostic value. Many of these studies have focused on bio-markers that feature in one or two pathways that are dysregulated in bladder cancer [48].
In the context of cell cycle regulation, the following markers have been examined in combination to predict bladder cancer outcome: p53, p21, Rb and/or p16 [49,50]. The undertaken studies suggested that incremental numbers of altered markers was independently correlated with significantly increased risk for bladder cancer recurrence, progression and mortality. Other comprehensive reviews have also commented on the combined prognostic utility of cell cycle regulation markers in bladder cancer [51].
Recent tissue microarray-based investigations are further facilitating this effort by increasing the throughput of protein-expression assays on primary bladder tumor tissues. These studies have demonstrated that interrogation of markers that are important for cell cycle regulation (p53, Rb, p21, p27 and/or cyclin E1) and apoptosis (Bcl-2, caspase-3, p53 and survivin) can be successfully accomplished [52–54]. Additional efforts are needed to profile alterations in crucial molecular determinants across several tumorigenic processes that can yield more comprehensive panels to predict disease outcome independent of standard clinicopathologic criteria.
Identification of prognostic marker panels
Even if markers are examined in combination, the true patho-physiology of bladder cancer may only be deciphered by investigating several pathways simultaneously in a medium- to high-throughput manner. Such panels may be generated at the epigenetic, genetic, transcriptomic, proteomic and metabolomic levels. Gene and transcriptome-level profiling are the most commonly applied approaches in bladder carcinoma patients.
There are two broad methodologies by which prognostic gene panels may be identified in disease processes: the global approach and the pathway-specific approach. While the global approach uses the whole coding sequence of the genome (or a significant portion of the coding sequence of the genome) to find marker panels that can predict bladder cancer outcome, pathway-specific approaches profile finite numbers of targets in a quantitative manner across several pathways.
The global approach
The decoding of the human genome has accelerated the research on identifying prognostic molecular markers for bladder cancer. The global approach interrogates the entire genome for alterations at the genetic and transcriptomic level in bladder cancer patients.
Routine microarray technology uses a high-throughput strategy to hybridize fluorescently labeled transcripts that are isolated from biological samples such as tumor specimens from bladder cancer patients. For this process, the isolated RNA is reverse transcribed to cDNA. This cDNA is transcribed to cRNA using nucleotides coupled with fluorescent molecules. The labeled cRNA is hybridized to the DNA microarray slide and the probe intensity is measured by the level of emitted fluorescence. Several studies have used such platforms to generate panels that can identify the presence or absence, and stage of disease [55–59]. Other studies have utilized this tool to perform molecular characterization of tumor subtypes and carcinoma in situ (CIS) [60]. More recent studies have also identified panels that can distinguish between molecular disease subgroups in terms of patient prognosis [61–64].
While the global approach can interrogate the entire coding sequence by a high-throughput automated system that promises a high discovery potential and selects panels in a relatively unbiased fashion, it usually results in a large panel of markers, which might result in some degree of false discovery. The utility of each panel therefore depends on the balance between the signature size and the clinical measure that it seeks to address (Figure 1). Furthermore, high-throughput platforms have some problems with reproducibility and are far more expensive than the pathway-specific approach.
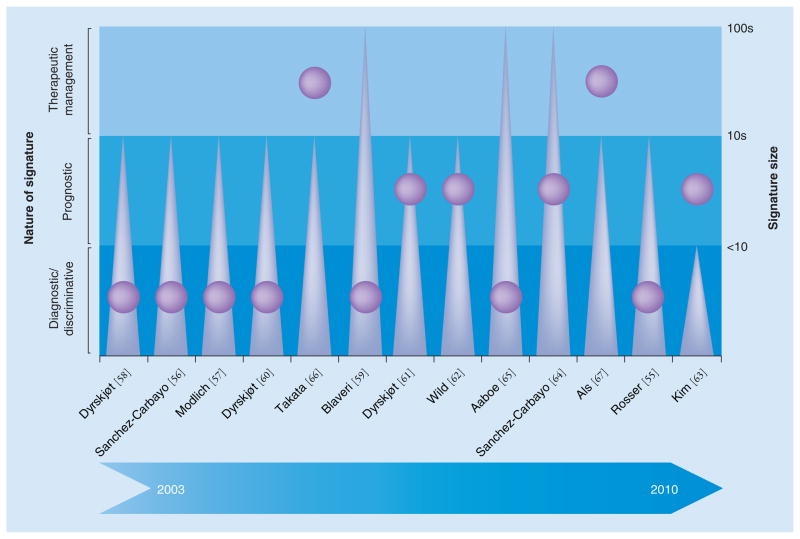
Figure 1. Subjective evaluation of the clinical utility of marker panels identified in different bladder cancer expression-profiling studies.
Studies have been identified by the last name of the first author and corresponding reference number on the horizontal axis. The major marker panel identified in each study has been classified by its primary clinical output (diagnostic/prognostic/therapeutic; purple circles corresponding to the shaded blue regions labeled on the left) and feature size (triangles corresponding to right vertical axis). Both metrics in combination influence the potential clinical utility of each signature.
Rosser et al. applied gene-expression profiling using a cDNA array on exfoliated urothelial cells from bladder washings [55]. A total of 46 patients with subsequently confirmed presence or absence of bladder cancer were prospectively investigated. Hierarchical clustering and supervised learning algorithms were used to classify samples on the basis of tumor burden. A panel of 319 differently expressed gene probes was associated with bladder cancer (p < 0.01). Visualization of protein interaction networks showed that VEGF and angiotensinogen were decisive markers for bladder cancer cells. Using supervised machine learning and a cross-validation approach, a 14-gene molecular classifier was built. With these 14 genes, an overall accuracy of 76% was achieved in distinguishing between patients with and without bladder cancer.
In another study, Sanchez-Carbayo et al. compared the expression profiles of early versus advanced bladder cancers using a cDNA microarray containing 17,842 genes and expressed sequence tags in 104 patients with a median follow-up of 18 months [56]. Hierarchical clustering segregated individuals with early-stage (40 patients) from invasive (64 patients) bladder cancer into two clusters. They were also successful in separating CIS from papillary superficial lesions within the early-stage tumors. The expression patterns of cytokeratin 20, neuropilin-2, p21 and p33ING1 were significantly associated with pathologic stage, tumor grade and altered Rb expression. Furthermore, p33ING1 expression levels were significantly associated with overall survival. The differentially expressed molecular targets were validated using immunohistochemistry on tissue microarrays. An analysis of significant transcripts revealed that genes encoding DEK and CD86 antigen were overexpressed in early-stage and invasive disease, respectively.
Modlich et al. also used a cDNA platform to investigate potential genes that contribute to disease progression in bladder cancer [57]. A total of 42 bladder tumors (22 non-muscle-invasive, 20 muscle-invasive) from 34 patients with adequate oncologic follow-up were analyzed using a 1185-gene filter-based cDNA array. Using a two-way clustering algorithm that used different subsets of gene-expression data, the tumors were classified according to their clinical outcome as non-muscle-invasive, muscle-invasive or metastasizing. Quantitative PCR (qPCR) was used to validate the array data for nine genes. The array data were further validated using oligonucleotide micro-arrays with 22,283 human gene fragments in a subset of three non-muscle-invasive- and six muscle-invasive tumor patients. Several gene clusters that characterized non-muscle-invasive and muscle-invasive tumors were identified.
With a 32-gene molecular classifier using a cross-validation approach, Dyrskjøt et al. were able to classify benign and muscle-invasive tumors from 66 biopsy samples using Affymetrix (CA, USA) oligonucleotide microarrays [58]. The classifier provided new predictive information on disease progression in Ta bladder cancers compared with conventional staging (p < 0.005). To delineate nonrecurring Ta tumors from frequently recurring cancers, the authors analyzed expression patterns in 31 tumors by applying a supervised learning classification methodology, with which 75% of samples were read correctly (p < 0.006). The gene-expression profiles characterizing each stage and subtype characterized their respective molecular properties, thereby potentially identifying new therapeutic targets.
A study by Blaveri et al. used expression microarray analysis to investigate 80 bladder tumors, nine bladder cancer cell lines and three normal bladder tissues [59]. cDNA microarrays with 10,368 human gene elements were used to analyze the samples. Unsupervised hierarchical clustering was able to separate the samples into groups of non-muscle-invasive (Ta and T1) and muscle-invasive (T2–T4) tumors. Supervised classification showed a 90.5% success rate in separating these two groups, when limited to a subset of 25 genes. The tissues were also separated into transitional versus squamous cell carcinoma subtypes (89% success rate), and good versus bad prognosis (78% success rate). The stage classifier was confirmed using data from an independent data set. By immunohistochemistry on tissue microarrays, the investigators were able to validate the value of cathepsin E and parathyroid hormone-related protein, biomarkers that were able to distinguish between non-muscle-invasive versus muscle-invasive tumors and transitional versus squamous cell carcinomas, respectively.
Aaboe et al. investigated gene-expression changes that occur during neoplastic transformation from normal to cancerous urothelium [65]. Using the Affymetrix platform, the authors reported on differentially expressed genes in Ta tumors compared with normal bladder urothelium, and on genes that were altered in high-grade tumors. The most significant expression differences were noticed in genes related to the cytoskeleton (keratin 7 and syndecan 1) and transcription (high mobility group AT-hook 1). In high-grade tumors, genes related to cell cycle control (CDK4) and transcription (jun d proto-oncogene) were altered. Keratin 7 overexpression was validated using western blot analysis in tumor tissues from bladder cancer patients.
Dyrskjøt et al. analyzed the tumor gene expression profiles of patients with non-muscle-invasive bladder cancer with and without concurrent CIS and those with muscle-invasive disease in a cohort of 41 patients using the Affymetrix U133A GeneChip™ platform [60]. Hierarchical clustering separated the non-muscle-invasive tumor samples according to the presence or absence of CIS. A few gene clusters were identified that had similar expression profiles across samples with non-muscle-invasive tumors with accompanying CIS and muscle-invasive disease. However, the authors were unable to identify any relationship between tumors with surrounding CIS and muscle-invasive disease using hierarchical clustering. The CIS-related profile was also noticeable in patients with grossly normal urothelium with surrounding CIS. A supervised learning approach was used to build a 16-gene molecular CIS classifier that was able to stratify non-muscle-invasive tumor samples according to the presence or absence of CIS with high accuracy.
For the prediction of clinical outcome, Dyrskjøt et al. performed another study to identify markers of bladder cancer progression using genome expression-profiling analysis with 59,619 genes and expressed sequence tags [61]. Non-muscle-invasive bladder tumors from 29 patients (13 without and 16 with disease progression) were used. Using a supervised learning approach, a 45-gene signature of disease progression was determined. The progression signature showed a significant correlation with clinical outcome in an independent test set (p < 0.03). The differentially expressed genes involved those that regulate apoptosis, cell differentiation and the cell cycle.
Another study on genetic changes relevant to bladder cancer progression was conducted on recurrent papillary tumors by Wild et al. [62]. A total of 67 bladder carcinomas (Ta–T2 tumors) and eight normal bladder specimens were profiled following laser microdissection. Hierarchical clustering did not show any significant differences between grade I and II papillary tumors. However, different gene-expression profiles were noticed between papillary and solid tumors. Progression-associated gene profiles were defined, and finally cathepsin E expression and a high Ki-67 labeling index of at least 5% were the only factors that correlated significantly with progression-free survival in patients with Ta tumors.
Kim et al. have recently employed high-throughput expression microarrays and qPCR validation on 272 bladder cancer specimens to identify prognosis-related gene classifiers [63]. Microarray analysis was carried out on 165 bladder cancer specimens used as a training cohort. The gene classifiers were analyzed with presence or absence of muscle invasion and prognosis. The authors validated selected gene classifiers using qPCR in the training and validation (107 patients) cohorts. In total, 97 genes related to disease progression were identified in patients with non-muscle-invasive bladder cancer. The eight-gene progression-related classifier in patients with non-muscle-invasive bladder cancer was closely correlated with progression in the validation cohort.
Sanchez-Carbayo et al. have documented a genetic signature that is characteristic of aggressive clinical behavior in bladder cancer [64]. A total of 105 bladder tumors were analyzed using oligonucleotide arrays (33 non-muscle-invasive and 72 muscle-invasive, as well as 52 normal tissues). Hierarchical clustering stratified normal urothelium, non-muscle-invasive and muscle-invasive tumors with 82.2% accuracy, and classified bladder cancers on the basis of clinical outcome. A predictive support vector machine algorithm rendered an 89% success rate for predicting tumor stage between non-muscle-invasive and muscle-invasive tumors, taking any of the top 250 differentially expressed genes. Accuracies of 82 and 90% were obtained for prediction of overall survival when considering patients with bladder cancer or only patients with muscle-invasive disease, respectively. A genetic signature of 174 probes was attributed to patients with nodal metastasized disease and poor survival. Independent analyses confirmed the association of this profile with the presence of positive lymph nodes and overall survival.
Takata et al. adopted genome-wide gene-expression profiling to identify predictive signatures of response to methotrexate, vinblastine, doxorubicin and cisplatin-based neoadjuvant chemotherapy in bladder cancer [66]. A cDNA microarray interrogating 27,648 genes was used to profile 27 muscle-invasive bladder cancers after they were laser microdissected; 14 predictive genes that were most differentially expressed between the responders and nonresponders were validated by qPCR. Using this 14-gene signature, the authors designed a qPCR-based system for potential clinical use in predicting chemotherapeutic response.
A similar approach was chosen by Als et al. to profile primary tumors from 30 patients with locally advanced or metastatic bladder carcinoma using Affymetrix HU133A GeneChips [67]. A total of 55 genes were found to be differentially expressed and highly correlated to survival time after chemotherapy. Two of the protein products from this panel (emmprin and survivin) were validated by immunohistochemistry on an independent cohort of 124 patients who received cisplatin-based chemotherapy. Multivariate analysis identified emmprin and survivin as independent prognostic markers for poor outcome and response to chemotherapy, along with the presence of visceral metastases.
Interestingly, the data sets used by Takata et al. and Als et al. were also used in a coexpression extrapolation algorithm to generate multivariate gene-expression models [68]. These models were initially based on in vitro drug sensitivities and microarray analyses of the NCI-60 cancer cell line panel. The gene-expression models effectively stratified tumor response and survival in bladder cancer patients treated with neoadjuvant chemotherapy independent of standard clinicopathologic parameters. The 3-year overall survival for those with favorable model scores was 81 versus 33% for those with less favorable scores (p = 0.002).
The pathway-specific approach
The data used to select markers for a pathway-specific approach may be generated by high-throughput techniques from global approaches, low-throughput profiling studies and in vitro experimentation. The major difference to the global approach is the more hypothesis-driven strategy. Quantification of gene expression can be performed using multiplexed qPCR, which represents the current gold standard method of validating expression profiling results [69].
Besides dealing with concise definitive gene panels that are usually highly reproducible across laboratories, the pathway-specific approach is also relatively inexpensive, making it more clinically applicable. However, the restricted number of biomarkers that may be interrogated limits the discovery potential of this methodology compared with the high-throughput approaches. Furthermore, the marker selection may be biased based on prior studies and the existing knowledge in the field. As described later, several studies have adopted this approach to profile alterations across several pathways that are commonly dysregulated in cancer (Figure 2).
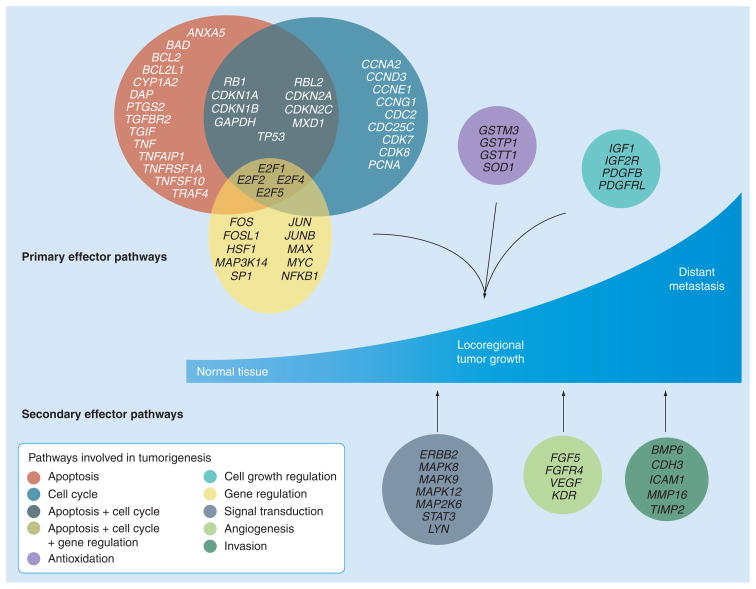
Figure 2. Examples of pathway-specific markers investigated in bladder cancer.
Mitra et al. [34,36] and Birkhahn et al. [32] have profiled genes across several broad pathways commonly deregulated in cancer on primary bladder tumors to develop molecular panels for detection of adverse events. The primary effector pathways of tumorigenesis include apoptosis, cell cycle, gene regulation, cell growth regulation and antioxidation. There is considerable overlap of markers among the first three pathways. pathways include signal transduction, angiogenesis and invasion.
Modified with permission from [34].
We have used this strategy to develop an objective method for detection of nodal metastasis from the molecular profiles generated from primary bladder tumor tissues using qPCR [34]. This study investigated 60 bladder cancer patients across all disease stages and five control tissues of normal urothelium. The cohort was divided into training and validation sets comprising node-positive and -negative cases. Using a genetic programming algorithm on expression values from a panel of 70 genes across eight broad pathways, classifier rules were generated that demonstrated an 81% accuracy on the validation set compared with pathologic nodal status. The rules showed a strong predilection for ICAM1, MAP2K6 and VEGFR2, resulting in gene-expression motifs that cumulatively suggested an expression pattern of ICAM1>MAP2K6>VEGFR2 for node-positive patients. Rules generated using only ICAM1, MAP2K6 and VEGFR2 were robust, with a single rule being 90% accurate when applied to the validation set.
We have also used this strategy to investigate gene signatures that can predict recurrence and progression in Ta tumors at first presentation, in order to be able to individualize the treatment of these patients [32]. This study used a 24-gene panel spanning across relevant cancer pathways to profile patients initially presenting with Ta grade 2–3 bladder carcinoma who belonged to one of three groups: no recurrence, recurrence or progression within 5 years of follow-up (16 patients in each group). CCND3 (p = 0.003) and HRAS (p = 0.01) were predictive for recurrence by univariate analysis. A multivariate model based on CCND3 expression showed 97% sensitivity and 63% specificity for recurrence. HRAS (p < 0.001), E2F1 (p = 0.017), Survivin (p = 0.038) and VEGFR2 (p = 0.047) were individually identified to be predictive for progression. A multivariate analysis based on HRAS, VEGFR2 and VEGF identified patients who progressed with 81% sensitivity and 94% specificity.
Another pathway-based profiling effort tried to identify molecular alterations associated with bladder cancer progression across all disease stages, which could potentially supplement TNM staging in predicting clinical outcome [36]. The expression levels of 69 genes involved in different cancer pathways were evaluated on bladder specimens from 58 bladder cancer patients. Expression levels of the same genes were also computed on normal urothelium tissues that were used as controls. By univariate analysis, six genes were found to be associated with time to recurrence and ten genes were associated with overall survival. Recursive partitioning analysis identified three genes that significantly predict recurrence, and three for overall survival. Based on the hypothesis that the most biologically relevant genes would predict both outcome measures, four genes (JUN, MAP2K6, STAT3 and ICAM1) were chosen for the final panel. A favorable versus unfavorable profile using this panel showed differences in 5-year recurrence and overall survival probabilities of 41 versus 88%, and 61 versus 5%, respectively (both p < 0.001). The prognostic potential of the four-gene panel was confirmed on an independent external data set (disease-specific survival; p = 0.039).
Conclusion
Molecular alterations across several tumorigenic pathways that are characteristically associated with bladder cancer have been broadly investigated by several groups. Advances in gene and protein expression-profiling technologies are now permitting the discovery of new biomarkers that can predict patient outcome and therapeutic response. However, the increasing wealth of literature in this field suggests that several pathways and molecules that can determine the clinical outcome have yet to be identified. Both global and pathway-specific approaches have their advantages and disadvantages, and can potentially be used in tandem to identify and validate new signatures of clinical importance.
Expert commentary
The current management of bladder cancer includes early detection, conservative-to-radical resection, close follow-up to monitor for progression, and accommodating lifestyle changes following surgery. Current indicators of prognosis include clinicopathologic staging criteria with limited objective parameters. While several individually prognostic molecules have been identified, there is a general lack of consensus on which markers can be employed in the clinical setting. The current trend is to move towards multiplexing marker assays in an effort to enhance their prognostic utility while better delineating the biologic course of disease progression. Initial results of these multimarker investigations have been encouraging. However, problems with reproducibility and variations between platforms remain, leading to limited overlap among panels that have been identified through various studies. Given that tumorigenesis is a multistep process involving dysregulation in multiple pathways, a concerted effort, possibly involving systems biology-based approaches, are needed to examine sequential molecular aberrations that eventually lead to the malignant phenotype, and contribute to disease progression and poor outcome.
Five-year view
Future management of bladder cancer will employ consensus marker panels that will be able to accurately predict prognosis and therapeutic response. However, in the short term, investigators have to overcome the impediments of profiling small cohorts and generating large panels of markers that have an admixture of immensely and negligibly informative molecules. While global profiling approaches have huge potential for the discovery of novel biomarkers, the issues regarding lack of reproducibility are disconcerting. Pathway-based approaches may not primarily be discovery tools, but can be leveraged toward hypothesis-driven investigations to generate and validate marker panels that are concise enough for clinical implementation. The ultimate aim with both approaches is to generate marker panels that have clinical utility while also providing biologic insight into the disease process. Retrospective single-/multi-institutional cohorts and prospective trials can then be used to validate such existing and novel marker panels for clinical implementation. Focused and validated panels can serve a dual purpose: predicting the outcome of individual patients based on their tumor’s molecular make-up and identifying potential targets for novel therapeutics, thereby eventually personalizing patient management.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial and competing interests disclosure
Studies described in this article were supported in part by NIH Grants CA-70903, CA-14089, CA-86871 and CA-103455, and National Cancer Institute Grant CA-71921. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Heney NM. Natural history of superficial bladder cancer. Prognostic features and long-term disease course. Urol Clin North Am. 1992;19(3):429–433. [PubMed] [Google Scholar]
- 3.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. Springer-Verlag; NY, USA: 2002. Urinary bladder; pp. 367–374. [Google Scholar]
- 4.Dutta SC, Smith JA, Jr, Shappell SB, Coffey CS, Chang SS, Cookson MS. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001;166(2):490–493. [PubMed] [Google Scholar]
- 5.Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163(6):1693–1696. [PubMed] [Google Scholar]
- 6.Murphy WM, Takezawa K, Maruniak NA. Interobserver discrepancy using the 1998 World Health Organization/International Society of Urologic Pathology classification of urothelial neoplasms: practical choices for patient care. J Urol. 2002;168(3):968–972. doi: 10.1016/S0022-5347(05)64553-3. [DOI] [PubMed] [Google Scholar]
- 7.Bol MG, Baak JP, Buhr-Wildhagen S, et al. Reproducibility and prognostic variability of grade and lamina propria invasion in stages Ta, T1 urothelial carcinoma of the bladder. J Urol. 2003;169(4):1291–1294. doi: 10.1097/01.ju.0000055471.78783.ae. [DOI] [PubMed] [Google Scholar]
- 8••.Mitra AP, Cote RJ. Molecular pathogenesis and diagnostics of bladder cancer. Annu Rev Pathol. 2009;4:251–285. doi: 10.1146/annurev.pathol.4.110807.092230. Comprehensive review of pathway-specific alterations in bladder cancer. [DOI] [PubMed] [Google Scholar]
- 9.Mitra AP, Birkhahn M, Cote RJ. p53 and retinoblastoma pathways in bladder cancer. World J Urol. 2007;25(6):563–571. doi: 10.1007/s00345-007-0197-0. [DOI] [PubMed] [Google Scholar]
- 10.Mitra AP, Lin H, Datar RH, Cote RJ. Molecular biology of bladder cancer: prognostic and clinical implications. Clin Genitourin Cancer. 2006;5(1):67–77. doi: 10.3816/CGC.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 11.Mitra AP, Cote RJ. Searching for novel therapeutics and targets: insights from clinical trials. Urol Oncol. 2007;25(4):341–343. doi: 10.1016/j.urolonc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.George B, Datar RH, Wu L, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007;25(34):5352–5358. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- 13•.Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331(19):1259–1264. doi: 10.1056/NEJM199411103311903. Study demonstrating that p53 nuclear accumulation corresponds with worse prognosis in bladder cancer. [DOI] [PubMed] [Google Scholar]
- 14.Sarkis AS, Dalbagni G, Cordon-Cardo C, et al. Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: a marker for disease progression. J Natl Cancer Inst. 1993;85(1):53–59. doi: 10.1093/jnci/85.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Serth J, Kuczyk MA, Bokemeyer C, et al. p53 immunohistochemistry as an independent prognostic factor for superficial transitional cell carcinoma of the bladder. Br J Cancer. 1995;71(1):201–205. doi: 10.1038/bjc.1995.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra AP, Lin H, Cote RJ, Datar RH. Biomarker profiling for cancer diagnosis, prognosis and therapeutic management. Natl Med J India. 2005;18(6):304–312. [PubMed] [Google Scholar]
- 17.Stein JP, Ginsberg DA, Grossfeld GD, et al. Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst. 1998;90(14):1072–1079. doi: 10.1093/jnci/90.14.1072. [DOI] [PubMed] [Google Scholar]
- 18.Mitra AP, Datar RH, Cote RJ. Molecular staging of bladder cancer. BJU Int. 2005;96(1):7–12. doi: 10.1111/j.1464-410X.2005.05557.x. [DOI] [PubMed] [Google Scholar]
- 19.Simon R, Struckmann K, Schraml P, et al. Amplification pattern of 12q13–q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene. 2002;21(16):2476–2483. doi: 10.1038/sj.onc.1205304. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Carbayo M, Socci ND, Kirchoff T, et al. A polymorphism in HDM2 (SNP309) associates with early onset in superficial tumors, TP53 mutations, and poor outcome in invasive bladder cancer. Clin Cancer Res. 2007;13(11):3215–3220. doi: 10.1158/1078-0432.CCR-07-0013. [DOI] [PubMed] [Google Scholar]
- 21.Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24(35):5552–5564. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 22.Mitra AP, Bartsch CC, Cote RJ. Strategies for molecular expression profiling in bladder cancer. Cancer Metastasis Rev. 2009;28(3–4):317–326. doi: 10.1007/s10555-009-9196-5. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto H, Shuin T, Torigoe S, Iwasaki Y, Kubota Y. Retinoblastoma gene mutations in primary human bladder cancer. Br J Cancer. 1995;71(4):831–835. doi: 10.1038/bjc.1995.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cote RJ, Dunn MD, Chatterjee SJ, et al. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998;58(6):1090–1094. [PubMed] [Google Scholar]
- 25.Chatterjee SJ, George B, Goebell PJ, et al. Hyperphosphorylation of pRb: a mechanism for RB tumour suppressor pathway inactivation in bladder cancer. J Pathol. 2004;203(3):762–770. doi: 10.1002/path.1567. [DOI] [PubMed] [Google Scholar]
- 26.Perabo FG, Kamp S, Schmidt D, et al. Bladder cancer cells acquire competent mechanisms to escape Fas-mediated apoptosis and immune surveillance in the course of malignant transformation. Br J Cancer. 2001;84(10):1330–1338. doi: 10.1054/bjoc.2001.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamana K, Bilim V, Hara N, et al. Prognostic impact of FAS/CD95/APO-1 in urothelial cancers: decreased expression of Fas is associated with disease progression. Br J Cancer. 2005;93(5):544–551. doi: 10.1038/sj.bjc.6602732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf HK, Stober C, Hohenfellner R, Leissner J. Prognostic value of p53, p21/WAF1, Bcl-2, Bax, Bak and Ki-67 immunoreactivity in pT1 G3 urothelial bladder carcinomas. Tumour Biol. 2001;22(5):328–336. doi: 10.1159/000050635. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Campora R, Davalos-Casanova G, Beato-Moreno A, et al. BCL-2, TP53 and BAX protein expression in superficial urothelial bladder carcinoma. Cancer Lett. 2007;250(2):292–299. doi: 10.1016/j.canlet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Pasin E, Josephson DY, Mitra AP, Cote RJ, Stein JP. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol. 2008;10(1):31–43. [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald JM, Ramchurren N, Rieger K, et al. Identification of H-ras mutations in urine sediments complements cytology in the detection of bladder tumors. J Natl Cancer Inst. 1995;87(2):129–133. doi: 10.1093/jnci/87.2.129. [DOI] [PubMed] [Google Scholar]
- 32•.Birkhahn M, Mitra AP, Williams AJ, et al. Predicting recurrence and progression of noninvasive papillary bladder cancer at initial presentation based on quantitative gene expression profiles. Eur Urol. 2010;57(1):12–20. doi: 10.1016/j.eururo.2009.09.013. Study using pathway-specific approach identifies genes predictive of recurrence and progression of Ta bladder tumors at first presentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia G, Kumar SR, Hawes D, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175(4):1245–1252. doi: 10.1016/S0022-5347(05)00736-6. [DOI] [PubMed] [Google Scholar]
- 34.Mitra AP, Almal AA, George B, et al. The use of genetic programming in the analysis of quantitative gene expression profiles for identification of nodal status in bladder cancer. BMC Cancer. 2006;6:159. doi: 10.1186/1471-2407-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephanou A, Brar BK, Knight RA, Latchman DS. Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ. 2000;7(3):329–330. doi: 10.1038/sj.cdd.4400656. [DOI] [PubMed] [Google Scholar]
- 36••.Mitra AP, Pagliarulo V, Yang D, et al. Generation of a concise gene panel for outcome prediction in urinary bladder cancer. J Clin Oncol. 2009;27(24):3929–3937. doi: 10.1200/JCO.2008.18.5744. Study using pathway-specific approach identifies a four-gene panel that predicts recurrence and survival of bladder cancer patients across all stages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiniakos DG, Mellon K, Anderson JJ, Robinson MC, Neal DE, Horne CH. c-jun oncogene expression in transitional cell carcinoma of the urinary bladder. Br J Urol. 1994;74(6):757–761. doi: 10.1111/j.1464-410x.1994.tb07121.x. [DOI] [PubMed] [Google Scholar]
- 38.Yao HQ, Peng Y, Zhong ZZ, He HX, Li ZH. Association of the expressions of platelet-derived growth factor receptor and c-Fos with the biological characteristics of bladder cancer. Di Yi Jun Yi Da Xue Xue Bao. 2004;24(2):177–179. [PubMed] [Google Scholar]
- 39.Youssef RF, Mitra AP, Bartsch G, Jr, Jones PA, Skinner DG, Cote RJ. Molecular targets and targeted therapies in bladder cancer management. World J Urol. 2009;27(1):9–20. doi: 10.1007/s00345-008-0357-x. [DOI] [PubMed] [Google Scholar]
- 40.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87(21):1603–1612. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 41.Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med. 2009;11:e26. doi: 10.1017/S1462399409001173. [DOI] [PubMed] [Google Scholar]
- 42.Theodoropoulos VE, Lazaris A, Sofras F, et al. Hypoxia-inducible factor 1α expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46(2):200–208. doi: 10.1016/j.eururo.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Theodoropoulos VE, Lazaris AC, Kastriotis I, et al. Evaluation of hypoxia-inducible factor 1α overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005;95(3):425–431. doi: 10.1111/j.1464-410X.2005.05314.x. [DOI] [PubMed] [Google Scholar]
- 44.Palit V, Phillips RM, Puri R, Shah T, Bibby MC. Expression of HIF-1α and Glut-1 in human bladder cancer. Oncol Rep. 2005;14(4):909–913. doi: 10.3892/or.14.4.909. [DOI] [PubMed] [Google Scholar]
- 45.Crew JP, O’Brien T, Bradburn M, et al. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res. 1997;57(23):5281–5285. [PubMed] [Google Scholar]
- 46.Bernardini S, Fauconnet S, Chabannes E, Henry PC, Adessi G, Bittard H. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder cancer. J Urol. 2001;166(4):1275–1279. [PubMed] [Google Scholar]
- 47.Grossfeld GD, Ginsberg DA, Stein JP, et al. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst. 1997;89(3):219–227. doi: 10.1093/jnci/89.3.219. [DOI] [PubMed] [Google Scholar]
- 48.Birkhahn M, Mitra AP, Cote RJ. Molecular markers for bladder cancer: the road to a multimarker approach. Expert Rev Anticancer Ther. 2007;7(12):1717–1727. doi: 10.1586/14737140.7.12.1717. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee SJ, Datar R, Youssefzadeh D, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22(6):1007–1013. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 50.Shariat SF, Tokunaga H, Zhou J, et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22(6):1014–1024. doi: 10.1200/JCO.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 51.Rosenblatt R, Jonmarker S, Lewensohn R, et al. Current status of prognostic immunohistochemical markers for urothelial bladder cancer. Tumour Biol. 2008;29(5):311–322. doi: 10.1159/000170878. [DOI] [PubMed] [Google Scholar]
- 52.Shariat SF, Zlotta AR, Ashfaq R, Sagalowsky AI, Lotan Y. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod Pathol. 2007;20(4):445–459. doi: 10.1038/modpathol.3800757. [DOI] [PubMed] [Google Scholar]
- 53.Shariat SF, Karakiewicz PI, Ashfaq R, et al. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer. 2008;112(2):315–325. doi: 10.1002/cncr.23162. [DOI] [PubMed] [Google Scholar]
- 54.Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8(2):128–136. doi: 10.1016/S1470-2045(07)70002-5. [DOI] [PubMed] [Google Scholar]
- 55.Rosser CJ, Liu L, Sun Y, et al. Bladder cancer-associated gene expression signatures identified by profiling of exfoliated urothelia. Cancer Epidemiol Biomarkers Prev. 2009;18(2):444–453. doi: 10.1158/1055-9965.EPI-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez-Carbayo M, Socci ND, Lozano JJ, et al. Gene discovery in bladder cancer progression using cDNA microarrays. Am J Pathol. 2003;163(2):505–516. doi: 10.1016/S0002-9440(10)63679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modlich O, Prisack HB, Pitschke G, et al. Identifying superficial, muscle-invasive, and metastasizing transitional cell carcinoma of the bladder: use of cDNA array analysis of gene expression profiles. Clin Cancer Res. 2004;10(10):3410–3421. doi: 10.1158/1078-0432.CCR-03-0134. [DOI] [PubMed] [Google Scholar]
- 58•.Dyrskjøt L, Thykjaer T, Kruhoffer M, et al. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet. 2003;33(1):90–96. doi: 10.1038/ng1061. Study using global approach classifies bladder tumors into disease stages. [DOI] [PubMed] [Google Scholar]
- 59.Blaveri E, Simko JP, Korkola JE, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11(11):4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 60•.Dyrskjøt L, Kruhoffer M, Thykjaer T, et al. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64(11):4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. Study using global approach identifies a signature that can predict the presence of carcinoma in situ. [DOI] [PubMed] [Google Scholar]
- 61.Dyrskjøt L, Zieger K, Kruhoffer M, et al. A molecular signature in superficial bladder carcinoma predicts clinical outcome. Clin Cancer Res. 2005;11(11):4029–4036. doi: 10.1158/1078-0432.CCR-04-2095. [DOI] [PubMed] [Google Scholar]
- 62.Wild PJ, Herr A, Wissmann C, et al. Gene expression profiling of progressive papillary noninvasive carcinomas of the urinary bladder. Clin Cancer Res. 2005;11(12):4415–4429. doi: 10.1158/1078-0432.CCR-05-0259. [DOI] [PubMed] [Google Scholar]
- 63.Kim WJ, Kim EJ, Kim SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010;9:3. doi: 10.1186/1476-4598-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24(5):778–789. doi: 10.1200/JCO.2005.03.2375. Study using global approach identifies a gene signature that predicts aggressive clinical behavior in bladder cancer. [DOI] [PubMed] [Google Scholar]
- 65.Aaboe M, Marcussen N, Jensen KM, Thykjaer T, Dyrskjøt L, Ørntoft TF. Gene expression profiling of noninvasive primary urothelial tumours using microarrays. Br J Cancer. 2005;93(10):1182–1190. doi: 10.1038/sj.bjc.6602813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Takata R, Katagiri T, Kanehira M, et al. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11(7):2625–2636. doi: 10.1158/1078-0432.CCR-04-1988. Study using global approach identifies a panel that can predict sensitivity of invasive bladder cancers to cisplatin neoadjuvant chemotherapy. [DOI] [PubMed] [Google Scholar]
- 67.Als AB, Dyrskjøt L, von der Maase H, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13(15 Pt 1):4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 68.Williams PD, Cheon S, Havaleshko DM, et al. Concordant gene expression signatures predict clinical outcomes of cancer patients undergoing systemic therapy. Cancer Res. 2009;69(21):8302–8309. doi: 10.1158/0008-5472.CAN-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zieger K. High throughput molecular diagnostics in bladder cancer – on the brink of clinical utility. Mol Oncol. 2008;1(4):384–394. doi: 10.1016/j.molonc.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]