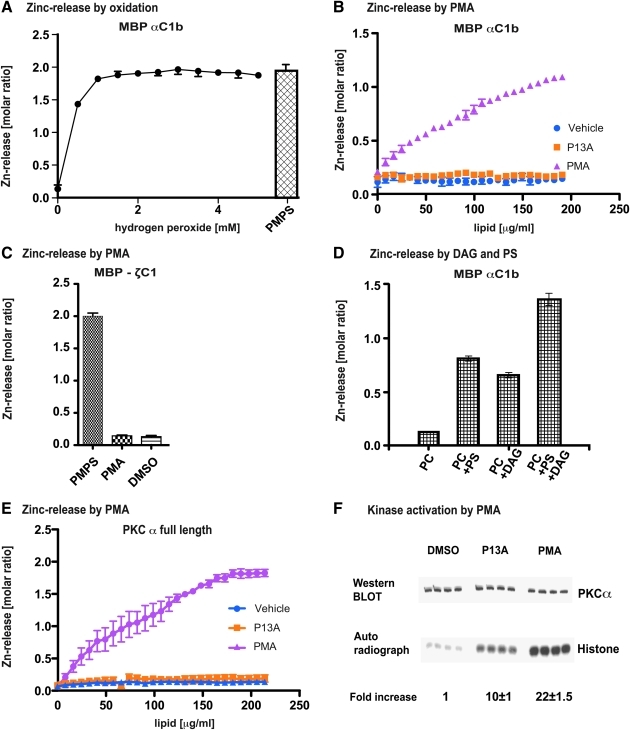
FIG. 1.
Zinc release by oxidation and lipid second messengers. (A) The zinc finger of maltose-binding protein (MBP)–protein kinase C α (PKCα) C1b is redox sensitive. Purified MBP-PKCα C1b fusion protein was incubated with increasing doses of H2O2 for 5 min at 37°C. Zinc release was monitored colorimetrically. Oxidation caused the release of two molar equivalents of Zn2+. PMPS is a reagent that forms thiol-mercaptide bonds with cysteines, thereby releasing all bound zinc from cysteine-containing zinc centers. Incubation of the MBP-PKCα C1b fusion protein with PMPS resulted in the release of two molar equivalents of Zn2+, indicating that both zinc-coordination centers were intact in the starting material. (B) The zinc finger of MBP-PKCα C1b responds to phorbol ester binding by releasing zinc. Purified MBP-PKCα C1b fusion protein was incubated with increasing doses of phorbol-12-myristoyl-13-acetate (PMA) or phorbol-13-acetate (P13A) for 5 min at 37°C. Zinc release was monitored colorimetrically. PMA caused the release of one zinc equivalent from MBP-PKCα C1b at maximum dose (200 μg/ml), whereas the weak phorbol agonist, P13A, was ineffective at similar concentrations. (C) The zinc finger of MBP-PKCζ C1 does not respond to phorbol ester binding. Purified MBP-PKCζ C1 fusion protein, containing a PMA nonbinding zinc finger, was incubated with increasing doses of PMA for 5 min at 37°C. Zinc release was monitored colorimetrically (titration not shown). The fusion protein failed to yield free Zn2+ upon stimulation with 200 μg/ml PMA. PMPS released two zinc equivalents. (D) The zinc finger of MBP-PKCα C1b responds to di-acyl-glycerol (DAG) and phosphatidyl-serine (PS) binding by releasing zinc. Both DAG and PS, applied with phosphocholine-based lipid vesicles, caused substantial zinc release from MBP-PKCα C1b fusion protein. The combination of DAG and PS released one Zn2+ equivalent, comparable to the molar ratio freed by PMA (as shown in B). (E) The zinc finger of full-length PKCα responds to phorbol ester binding by releasing zinc. Purified full-length PKCα, expressed in insect cells, was incubated with increasing doses of PMA or P13 A for 5 min at 37°C. Zinc release was monitored colorimetrically. PKCα responded to stimulation with PMA, but not P13A, and released at 200 μg/ml PMA two of its four zinc equivalents. Vehicle control was negative. (F) Full-length PKCα was activated by phorbol ester. Purified full-length PKCα, expressed in insect cells, was incubated with 200 μg/ml PMA or P13 A for 5 min at 37°C. Kinase activity was determined by measuring phosphotransferase capacity with histone as substrate. Upon stimulation with PMA full-length PKCα gained 22-fold higher phosphotransferase activity, whereas P13A produced less than half of this activity (p > 0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).