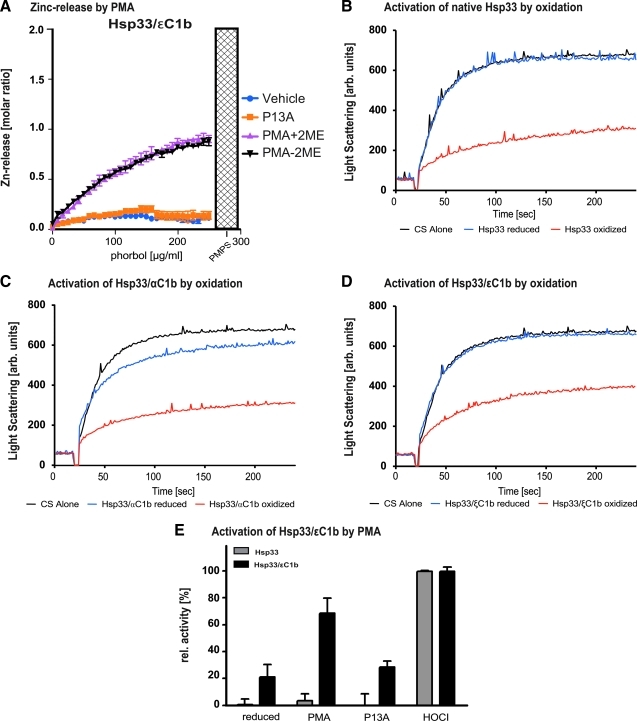
FIG. 2.
Chaperone activity of heat-shock protein-33 (Hsp33)-PKC chimeras is regulated by oxidation and by second messengers. (A) The Hsp33/ɛC1b reporter protein responds to PMA by half-maximal zinc release. Purified Hsp33/ɛC1b protein was titrated with PMA or P13A under the conditions described in (B). This resulted at maximum PMA concentration (200 μg/ml) in the release of two molar equivalents. PMPS yielded the expected four zinc equivalents. The PMA effect was not mediated by oxidation since the presence of β-mercaptoethanol did not diminish PMA effectiveness. P13 A did not cause zinc release. Chaperone activity of native Escherichia coli Hsp33 (B), the chimeric Hsp33/αC1b protein (C), or the chimeric Hsp33ɛC1b mutant protein (D). The influence of these proteins on the aggregation of chemically denatured citrate synthase (CS) at 30°C was tested. Light scattering of CS in the absence of any chaperones (black traces), in the presence of reduced, inactive chaperones (blue traces), or in the presence of oxidized chaperones, which were treated with 2 mM H2O2 at 43°C for 3 h (red traces), was monitored. (E) Effect of second messengers on the chaperone activity of Hsp33 and the chimeric Hsp33ɛC1b mutant protein. The reduced proteins were incubated in PMA, P13A, or HOCl for 3 h at 30°C. Then, the proteins were diluted 1:166-fold into the assay buffer and their effects on the aggregation of chemically denatured CS was determined as described. The residual concentrations of PMA, P13A or HOCl present in the assay buffer, did not have any significant effect on the light scattering signal of CS alone. Presence of PKCɛ C1b zinc finger conferred responsiveness to PMA at 30°C, but not to P13A. Native Hsp33 was not responsive to PMA. Both native and chimeric Hsp33 proteins were activated by hypochlorite (HOCl). Results presented are representative of at least three repeat experiments.