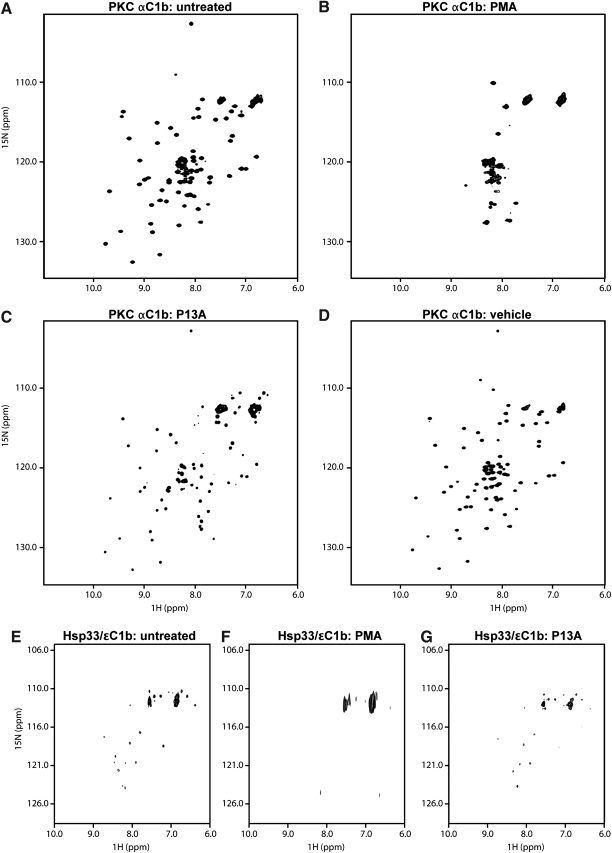
FIG. 3.
Zinc release and chaperone activation are associated with structural changes in the zinc-finger fold. (A-D) The zinc-finger folds of PKCα C1b loose their structure upon exposure to phorbol ester. (A) The HSQC spectrum of [U-15N] PKCαC1b (250 μM) shows a well-structured fold comparable to that of published spectra [14]. (B) After stimulation with PMA (protein:PMA ratio = 1:2.6), some precipitation occurred. However, after removal of aggregates the spectrum of the supernatant showed a disordered structure. (C) Treatment with P13A (1:2.6 molar ratio) caused only minor shifts. (D) Treatment with vehicle (dimethyl sulfoxide) caused no discernible shifts. (E) The chimeric Hsp33/ɛC1b protein was uniformly labeled with [15N]L-cysteine. The HSQC spectrum of the untreated protein (100 μM) revealed signals from six cysteine residues, and additional lower intensity signals arising from scrambling of the 15N label. (F) PMA treatment (protein:PMA, 1:1.3) resulted in some precipitation, as in (B). The HSQC spectrum of the supernatant (∼80 μM) showed broadening of signals suggesting loss of structure. (G) The weak phorbol agonist, P13A, while eliciting shifts of some cysteine residues (shown at 1:2.6 molar ratio), possibly due to secondary rearrangement in Hsp33, retained the six core cysteines in the fixed positions, indicating a structurally intact zinc finger.