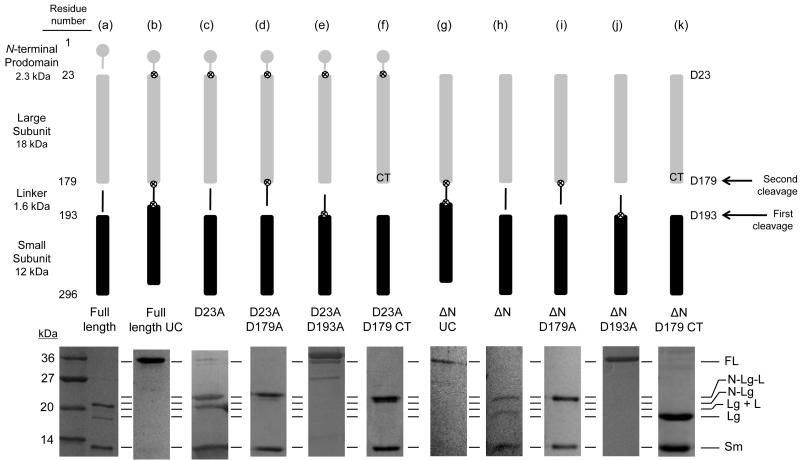
Fig. 1.
Caspase-6 cleavage site-blocking variants. Cartoon representation of expression constructs showing cleavable (gap) or blocked ⊗ caspase cleavage sites (upper panel). Symbols for protein domains include prodomain (gray circle), large subunit (gray bar), intersubunit linker (black line), and small subunit (black bar). Aspartic acid residues mutated to alanine are depicted as ⊗. Residue numbers for each domain are indicated at the left of the upper panel. SDS-PAGE analysis of expressed constructs matured in E. coli cells and the observed cleavage patterns (lower panel). (a) Full-length caspase-6. (b) Full-length uncleavable caspase-6 variant (FLUC) D23A/D179A/D193A completely blocks zymogen processing. (c) D23A. (d) D23A/D179A. (e) D23A/D193A. (f) D23A/D179 Constitutive Two-chain (CT) variant expresses the prodomain-large subunit protein inclusive of residue 179 independently of the small subunit, which is expressed from an introduced ribosome-binding site and start codon at residue 193. (g-k) Expression constructs similar to b-f but lack the coding sequence for the N-terminal prodomain. FL= Full Length; N = N-terminal prodomain; Lg = Large subunit; Sm = Small subunit, L = Intersubunit linker. The order of cleavage events leading to activation is indicated by arrows on the right.