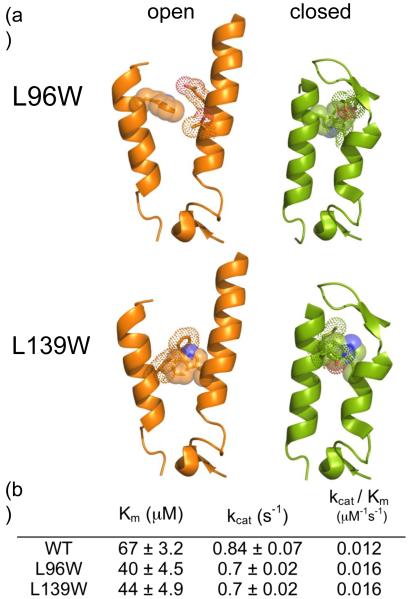
Fig. 5.
Mutants to probe the unique 90’s helix conformation. (a) Models of the 90’s and 130’s helices from caspase-6 (orange) in the open conformation and caspase-7 (green) in closed conformation. Caspase-6 positions 96 and 139 are shown mutated from Leu to Trp. Caspase-6 L96 and L139 correspond to residues M116 and L162 respectively in caspase-7. L96 and L139 can accommodate at least one rotomer of Trp in the open conformation, but no rotomers of Trp in the closed conformation, as evidenced by the observed clashes between the introduced Trp and adjacent side chains. (b) The kinetic parameters for caspase-6 ΔN D179A (WT), ΔN L96W D179A (L96W) and ΔN L139W D179A (L139W).