Table 1.
Oxidation level dependent anti-diastereoselectivity in ruthenium catalyzed couplings of allene 1c.
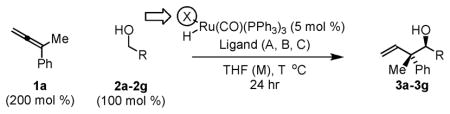 | |||||
|---|---|---|---|---|---|
| Entry | X | Alcohol | R | Ligand, T (M) | Yield (dr) |
| 1 | OMes | 2a | p-NO2Ph | A, 50 °C (1 M) | 3a, 84% (>20:1)d |
| 2 | Cl | 2b | p-CF3Ph | A, 40 °C (0.5 M) | 3b, 85% (4:1) |
| 3 | Cl | 2c | Ph | A, 60 °C (0.5 M) | 3c, 99% (6:1) |
| 4 | Cl | 2d | 2-Furyl | A, 60 °C (0.5 M) | 3d, 99% (10:1) |
| 5 | Cl | 2e | HC=CHPh | A, 60 °C (0.2 M) | 3e, 99% (5:1) |
| 6 | Cl | 2f | n-Hexyl | A, 75 °C (1 M) | 3f, 77% (>20:1)c,d |
| 7 | Cl | 2g | (CH2)3OBn | A, 75 °C (1 M) | 3g, 67% (>20:1)c,d |
 | |||||
|---|---|---|---|---|---|
| Entry | X | Alcohol | R | Ligand, T (M) | Yield (dr) |
| 8 | O2PBinol | 2a | p-NO2Ph | A, 75 °C (1 M) | 4a, 97% (16:1) |
| 9 | O2PBinol | 2b | p-CF3Ph | A, 75 °C (1 M) | 4b, 93% (>20:1) |
| 10 | O2PBinol | 2c | Ph | A, 95 °C (1 M) | 4c, 85% (17:1) |
| 11 | O2PBinol | 2d | 2-Furyl | A, 95 °C (1 M) | 4d, 71% (15:1) |
| 12 | O2PBinol | 2e | HC=CHPh | A, 95 °C (1 M) | 4e, 94% (14:1) |
| 13 | Cl | 2f | n-Hexyl | A, 95 °C (1 M) | 4f, 86% (4:1)c,d |
| 14 | Cl | 2g | (CH2)3OBn | A, 95 °C (1 M) | 4g, 69% (4:1)c,d |
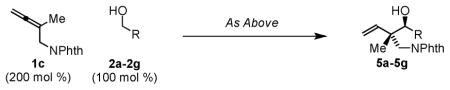 | |||||
|---|---|---|---|---|---|
| Entry | X | Alcohol | R | Ligand, T (M) | Yield (dr) |
| 15 | Cl | 2a | p-NO2Ph | C, 95 °C (0.5 M) | 5a, 83% (10:1) |
| 16 | Cl | 2b | p-CF3Ph | C, 95 °C (0.5 M) | 5b, 65% (14:1) |
| 17 | Cl | 2c | Ph | B, 75 °C (0.5 M) | 5c, 99% (8:1)d |
| 18 | O3SCam. | 2d | 2-Furyl | A, 85°C (1 M) | 5d, 84% (7:1) |
| 19 | Cl | 2e | HC=CHPh | B, 85°C (0.1 M) | 5e, 99% (5:1)d |
| 20 | Cl | 2f | n-Hexyl | A, 95°C (1 M) | 5f, 93% (>20:1)c,d |
| 21 | Cl | 2g | (CH2)3OBn | A, 85°C (1 M) | 5g, 72% (>20:1)d |
Yields are of isolated material. Diastereoselectivity was determined via 1H NMR analysis of crude reaction mixtures. See supporting information for further experimental details.
Ligands: A = dippf (5 mol %); B = dCypf, (5 mol%); C = PPh2Cy (15 mol %).
Three equivalents of allene.
48 hours.
