Table 1.
Kinetic Constants for the ADH3-Mediated Oxidation of NAE Substrates with Acyl Chains Containing 2-12 Carbon Atoms.
| Substrate | 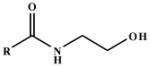 |
KM (mM) | VMAX (μmol/min/mg) | VMAX/KM (sec−1 M−1) |
|---|---|---|---|---|
| N-Acetylethanolamine | R = CH3 | (4.5 ± 0.4) × 102 | 1.9 ± 0.059 | 2.7 |
| N-Propionylethanolamine | R = CH3CH2 | 79 ± 3.8 | 1.9 ± 0.026 | 15 |
| N-Butyrylethanolamine | R = CH3(CH2)2 | 46 ± 5.4 | 1.7 ± 0.052 | 23 |
| N-Hexanoylethanolamine | R = CH3(CH2)4 | 9.5 ± 0.36 | 1.6 ± 0.018 | 110 |
| N-Octanoylethanolamine | R = CH3(CH2)6 | 5.8 ± 0.38 | 1.5 ± 0.028 | 160 |
| N-Decanoylethanolamine | R = CH3(CH2)8 | 0.32 ± 0.029 | 0.57 ± 0.024 | 1.1 × 103 |
| N-Lauroylethanolamine | R = CH3(CH2)10 | 0.033 ± 0.0061 | 0.14 ± 0.0088 | 2.7 × 103 |
| 1-Octanol | CH3-(CH2)6-CH2-OH | 0.050 ± 0.0130 | 2.5 ± 0.16 | 3.2 × 104 |
| Cinnamyl alcohol | C6H6HC=CHCH2OH | 0.035 ± 0.0033 | 4.0 ± 0.058 | 7.2 × 104 |
