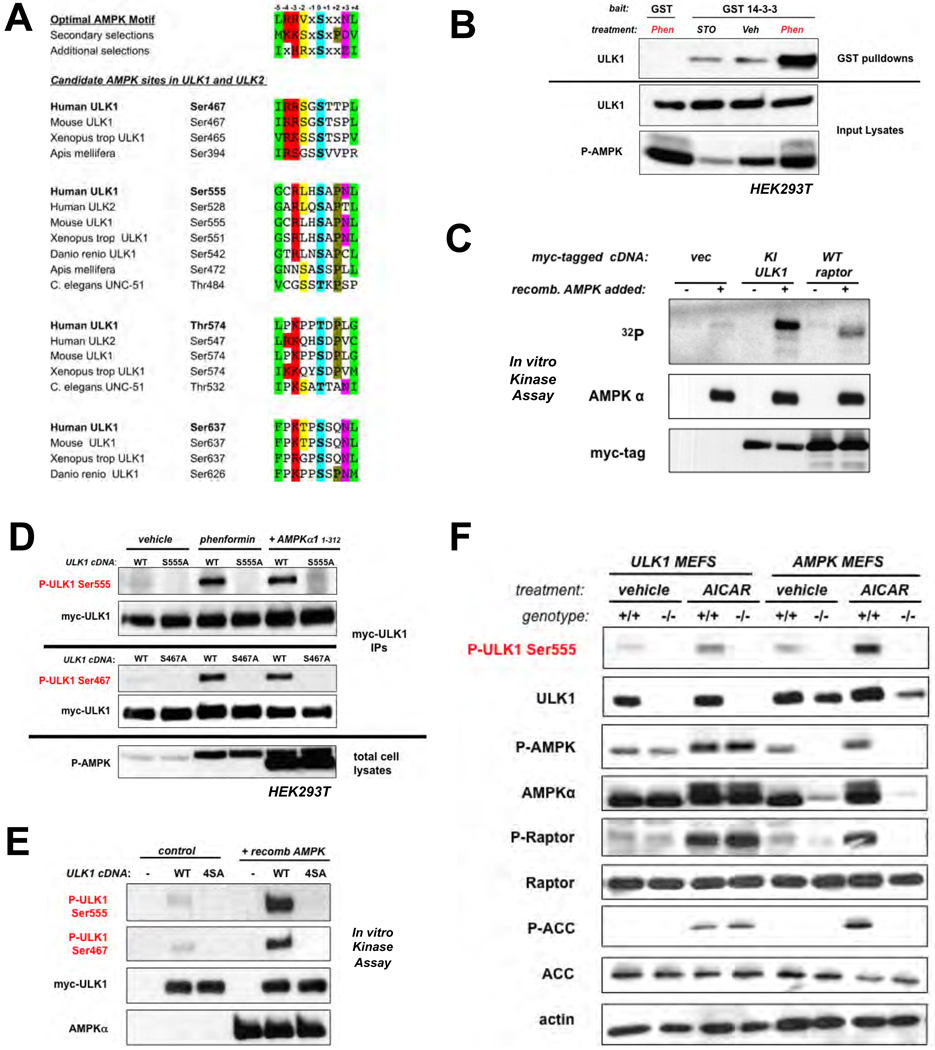
Fig. 1.
ULK1 is a conserved substrate of AMPK. (A) Clustal alignment of four conserved sites in ULK1 and two sites in ULK2 matching the optimal AMPK substrate motif. (B) ULK1 and GST or GST-14-3-3 expression vectors were transfected into human embryonic kidney (HEK)293T cells, and placed in media containing 20µM STO-609 (STO), vehicle (veh), or 5mM phenformin (Phen) for 1h. Cell lysates and GST pulldowns were immunoblotted as indicated. (C) In vitro kinase assays with myc-tagged catalytically inactive (KI: K46I) ULK1 or myc-tagged wild-type raptor which were immunoprecipitated from HEK293T cells and used as substrates for purified active AMPK in the presence of 32P-γ-ATP. (D) HEK293T cells transfected with myc-tagged wild-type ULK1 or indicated Serine-to-alanine ULK1 mutants were treated with either vehicle or 1mM phenformin for 1h, or were co-transfected with a constitutively active AMPKα1 (aa1-312) mammalian expression vector (11). Proteins from lysates were immunoblotted with phospho-specific antibodies as indicated. (E) In vitro kinase assays using myc-ULK1 and purified AMPK as above. Phosphorylation of myc-ULK1 detected by immunoblotting with indicated phospho-specific antibodies. (F) Primary murine embryonic fibroblasts (MEFs) were treated with 2mM AICAR or vehicle for 1h. Lysates immunoblotted as indicated including detection of endogenous ULK1 P-Ser555