Abstract
Ecological networks are complexes of interacting species, but not all potential links among species are realized. Unobserved links are either missing or forbidden. Missing links exist, but require more sampling or alternative ways of detection to be verified. Forbidden links remain unobservable, irrespective of sampling effort. They are caused by linkage constraints. We studied one Arctic pollination network and two Mediterranean seed-dispersal networks. In the first, for example, we recorded flower-visit links for one full season, arranged data in an interaction matrix and got a connectance C of 15 per cent. Interaction accumulation curves documented our sampling of interactions through observation of visits to be robust. Then, we included data on pollen from the body surface of flower visitors as an additional link ‘currency’. This resulted in 98 new links, missing from the visitation data. Thus, the combined visit–pollen matrix got an increased C of 20 per cent. For the three networks, C ranged from 20 to 52 per cent, and thus the percentage of unobserved links (100 − C) was 48 to 80 per cent; these were assumed forbidden because of linkage constraints and not missing because of under-sampling. Phenological uncoupling (i.e. non-overlapping phenophases between interacting mutualists) is one kind of constraint, and it explained 22 to 28 per cent of all possible, but unobserved links. Increasing phenophase overlap between species increased link probability, but extensive overlaps were required to achieve a high probability. Other kinds of constraint, such as size mismatch and accessibility limitations, are briefly addressed.
Keywords: Arctic, climate, Mediterranean shrubland, mismatch, pollination, seed dispersal
1. Introduction
A fundamental scientific paradigm is to observe natural phenomena and attempt to interpret them. Here we take a different approach by focusing upon what we do not see in nature and try to explain why. We begin by taking a stroll in nature with all its flowering plants and animals, of which some are observed to visit flowers for nectar and alongside service as pollinators, and others eat fruit and act as seed dispersers. However, not all animal visitors make links to all plant species. Some links are present (i.e. they are observed by us) whereas others remain unobserved. Species and their links form extensive networks [1]—for example, mutualistic networks between plants and their pollinators or seed dispersers—and such visualizations of nature's complexity reveal that, by far, most potential links in nature remain unobserved.
The process of sampling interactions is cumulative: observation time and/or samples (e.g. visits, pollen loads, faecal samples) are accumulated, and interactions are recorded. Unobserved links may be either missing from the sampling or forbidden (electronic supplementary material, S1). Sooner or later, missing links will be noted if sampling effort is extended, although some may be very rare. Thus, monitoring links is analogous to any biodiversity sampling (i.e. species inventory [2]) and is subject to similar methodological shortcomings, especially sampling species and links of low abundance. Forbidden links, on the other hand, will not be observed, irrespective of abundance and sampling effort [3]—that is, some pairwise interactions do not occur in nature because of linkage constraints. However, if a forbidden link (e.g. in a mutualistic network) in fact is observed, one of the interacting mutualists behaves as a cheater, such as when a short-tongued bee robs a long corolla-tubed plant for nectar [4], or when a gape-limited frugivore pecks the pulp of large fruits and drop the seeds of fruit it cannot handle and ingest [5]. Linkage constraints may be spatio-temporal uncoupling, size or reward mismatching, foraging constraints, physiological–biochemical constraints, etc. [6–13]. Spatio-temporal uncoupling means that species do not co-occur in space or time. Temporal uncoupling, for example, takes place when phenophases of potentially interacting species do not overlap. Thus, spatio-temporal uncoupling precedes other constraints and prevents their expression. The original match/mismatch hypothesis, coined by Cushing [14], focused upon temporal uncoupling of plankton blooms as a function of climate, but the idea has deeper roots in ecology [15] and outside biology as well (e.g. [16]). Size mismatching explains many forbidden links (e.g. in plant–hummingbird networks [9,17], plant–insect networks [12,18] and plant–frugivore interactions [2]). Foraging constraints are determined by rates of search and handling of, for example, fruits [19], and biochemical constraints are operating if a frugivore is unable to process secondary compounds in fruit pulp. Even a multiple-trait or multiple-constraint model may be needed to make solid predictions about link patterns in real-world networks [20]. However, Kallimanis et al. [21] have also suggested a neutral model based on variation in species phenophase and abundance. They showed how, through a purely stochastic process, it captured the topological features of a large real pollination network.
As link biologists and networkers, our overall goal is to reduce the number of missing links in our interaction inventories and explain the forbidden ones. In this study, (i) we estimated the numbers of observed and forbidden links in three mutualistic networks (and we discuss their potential causes); (ii) we explained to what extent forbidden links remained unobserved owing to phenological uncoupling; and (iii) we explored the effect of phenological overlap on linkage probability.
2. Material and methods
(a). Study networks, sites and periods
We used data from one pollination/flower-visitation network and two seed-dispersal/frugivory networks [2,11]. Flower visitors and frugivores were defined as pollinators and seed dispersers, respectively. The networks included all observed species and their interactions within the study areas. Number of missing links was reduced by (i) extending sampling time to a full season and (ii) using both a phyto-centric and a zoo-centric approach to monitor links, i.e. flower-/fruit-visit data and data on pollen on the body surface of flower visitors and seeds in faeces of frugivores, respectively ([22]; the ‘smoking gun’ method sensu [23]). As in any biodiversity monitoring, accounting for sampling effects when monitoring interactions has long been recognized as a fundamental aspect [6], and this two-sided approach was recommended by Blüthgen [24]; also see [22,25] to reduce effects of observation bias.
(i). Pollination network
The study site was a 0.5 × 0.5 km area of heathland, river banks and snow beds near the Zackenberg Research Station, northeast Greenland. An area of that size was needed to cover most of the different habitats, including late-melting snow beds. The study period included a full season from June to August 1996 (i.e. from when the last snow melted and till the first snow fell [11]). The study was repeated in 1997. In general, we suggest that constraints are studied within ‘the lifespan of a network’. Every new season should be one study entity, because constraints are not just site-specific but also time-specific. This is easy to do at high latitudes where the season is distinct, but difficult or even impossible at lower latitudes.
(ii). Seed-dispersal networks
The first study site, Hato Ratón (HR), in the lowlands near Seville (southwest Spain), was tall scrubland of sclerophylous shrubs and treelets (32 m a.s.l.) extending over 130 ha [2,26]. In our construction of the seed-dispersal networks, we had to pool the data over several years. The study period at HR lasted from December 1980 to March 1983. The second site, Nava de las Correhuelas (NC), Sierra de Cazorla, Jaén (southeast Spain), was montane pine forest and rocky slopes with deciduous species [27]. The study period lasted from April 1988 to March 2004.
(b). Link monitoring
(i). Pollination network
We used two kinds of link ‘currency’: a visit link was recorded whenever a pollinator species was observed to visit the flowers of a plant species, and a pollen link was recorded if pollen grains of a plant species were found on the body surface of a pollinator species. An increase in the use of one of the currencies is expected to reduce the effect of the use of the other. However, using two kinds of currency may be a methodological shortcut to detect more missing links with less effort. Observations of pollinator visitation were made at all flowering plant species (24 or fewer simultaneously flowering species) on all sunny and calm days from 09.00 to 17.00 h. Each daily observation census at a plant species lasted 20 min at each of two individuals. Thus, observation time per plant species was independent of plant species abundance (but not of phenophase length) and our sampling-accumulation analysis showed that links were sampled sufficiently (electronic supplementary material, S2). Whenever possible, flower-visiting insects were caught and stored individually in 70 per cent alcohol for later examination of their body-surface pollen load. In total, 1245 individuals (or 21 per cent of all visitors) were sampled, including representatives of all species. Since pollen grains may stay on the body of an insect for days, pollen gives more robust, cumulative link evidence and also detects interactions between rare species [22]. Consequently, we may find pollen from a plant species on an insect species on a day when their phenophases do not overlap. Our dataset had a few examples of that. Insects may also collect pollen from individual plants still in flower, but growing outside the study area. The same argument runs for the dispersal networks. Analysis of body-surface pollen load followed a protocol from Olesen & Warncke [28]. Pollen data are not corrected for variation in species abundance.
(ii). Seed-dispersal networks
Here, we only used one link currency at each study site. At NC, we used focal plant observations (data on handling fruit, seeds or remains of pulp; 8378 feeding records; 50–100 h of observation per plant species; i.e. a phyto-centric approach), and at HR we used analysis of frugivore diets (faeces from birds captured in mist nets; 3504 samples; i.e. a zoo-centric approach). This latter approach does not correct for variation in species abundance. A visit link was recorded during transect censuses throughout the study area at NC, whenever a frugivore species was observed to visit a plant species and pick a fruit. Observation time spent per species varied [27]. Fruit-pulp remains links were recorded from faecal samples obtained from mist-netted birds or directly sampled in the field at HR. Faeces were stored dry and later examined for seed and fruit-exocarp tissue, which has characteristic cell shapes, etc. This procedure made it possible to identify these plant parts to species [26]. Relying only on identification of seeds tends to severely underestimate number of species consumed, since seeds, compared with exocarps, typically have a shorter gut retention time and/or are more quickly regurgitated. Finally, this was complemented with field sampling of faeces of carnivorous mammals and bird species rarely captured in mist nets, e.g. Cyanopica cyanus, Sturnus unicolor and S. vulgaris. In our analysis of linkage constraints, we also used foraging and morphological data about birds and fruit extracted from [2,29].
(c). Recording of phenophases
(i). Pollination network
Flowering phenophase of a plant species spans the time from when the first individual of the species at the study site initiates flowering until the last individual has ceased to flower. Thus, pollen load data were not used in our estimation of phenophase length. Likewise, phenophase of a pollinator species spans the time from when the first individual is observed to visit a flower until the last individual is seen in a flower. This latter definition must give a minimum estimate of phenophase, since insects most probably were active some days before and after the recorded phenophase. However, during these days, the species must have a very low abundance and their linkage probability was assumed negligible. Our definition of phenophase is populational, in contrast to individual phenophase, which we do not have any data about. We expect species abundance and populational phenophase to be positively correlated [11]. A phenophase may be as brief as 1 day (if, for example, a species mainly lives outside the study area and one of its individuals only enters the area once).
(ii). Seed-dispersal networks
Fruiting phenophases were recorded from marked plants along transects (see [26] and references therein; [30]) and checked periodically (weekly or biweekly censuses) for the presence of ripe fruits. A fruiting phenophase of a plant species spans from the recording of the first ripe fruit until no fresh ripe fruits are present on any plants. Phenophases of frugivores were estimated from weekly or biweekly direct censuses along line transects and independent of the censuses for feeding records. At HR, we ran weekly mist netting [31] and estimated frugivore phenophases from these data combined with results of direct censuses during line-transect counts.
3. Results
(a). Observed and missing links
(i). Pollination network
A flower-visitation matrix was constructed, depicting all links observed during the entire season between all pollinator and plant species (electronic supplementary material, S3). A total of A = 61 insect species were observed to visit flowers of P = 31 plant species. Number of species links was I = 286, and matrix connectance C = 100 I/(AP) = 15 per cent (table 1). Thus, the number of unobserved links became AP − I = 1605 and their percentage was 100 – C = 85 per cent. Since we only made pollinator observations at each plant species for 40 min daily, observation time might be insufficient. However, a sampling-accumulation analysis of visitation data showed that we had robustly estimated the number of actual interactions occurring, and that sampling was sufficient to estimate the interaction matrix (electronic supplementary material, S2).
Table 1.
Mutualistic networks. A, number of animal species; P, number of plant species; I, number of links; C = 100 I/(AP), connectance; F, number of forbidden links; and M, number of missing links.
| pollination network (1996) | observed links |
|||
|---|---|---|---|---|
| A | P | I | C(%) | |
| (1) visit links | 61 | 31 | 286 | 15.1 |
| unobserved links as % of AP | ||||
| (2) missing links M sampled as pollen-load data | 98 | 5.2 | ||
| (3) total observed links = (1) + (2) | 384 | 20.3 | ||
| (4) forbidden links F = AP − (3) = (5) + (6) | 1507 | 79.7 | ||
| (5) owing to phenological uncoupling | 530 | 28.0 | ||
| (6) owing to other constraints | 977 | 51.7 | ||
| observed links |
||||
| seed-dispersal networks (pooled for all study years) | A | P | I | C(%) |
| (1) observed links | ||||
| HR | 17 | 16 | 141 | 51.8 |
| NC without mammals | 22 | 25 | 131 | 23.8 |
| NC with mammals | 25 | 25 | 156 | 25.0 |
| unobserved links as % of AP | ||||
| (2) forbidden links F = AP − (1) = (3) + (4) | ||||
| HR | 131 | 48.2 | ||
| NC without mammals | 419 | 76.2 | ||
| NC with mammals | 469 | 75.0 | ||
| (3) owing to phenological uncoupling | ||||
| HR | 68 | 25.0 | ||
| NC without mammals | 135 | 24.5 | ||
| NC with mammals | 135 | 21.6 | ||
| (4) owing to other constraints | ||||
| HR | 63 | 23.2 | ||
| NC without mammals | 284 | 51.7 | ||
| NC with mammals | 334 | 53.4 | ||
Still, some links, which could not be detected by our visit-observation protocol, might be missing. These might be detected by extending the observation time or by using other sources of information, such as data on pollen load on the surface of pollinators. We used the latter approach and identified pollen grains on sampled insect specimens to species. These data were pooled for the entire season. This pollen matrix included 48 pollen-carrying insect species, 29 plant species and 232 links, of which 98 were new (i.e. not seen in the visitation matrix; table 1). Thus, these M = 98 links were real and were missing from the visitation network. If we had extended our observation time, we would most certainly have observed some of these links. Number of links in the combined visitation–pollen matrix now added up to 384 links and C increased to 20 per cent (table 1). The overlap between visit and pollen links was only 35 per cent (=134/(98 + 286)), confirming the value of using more than one kind of link currency. In spite of this additional sampling effort, 1507 (=61 × 31 −(98 + 286)) or 80 per cent of all potential links remained unobserved. Most of these unobserved links might be attributable to biological causes (forbidden links F, in our definition; electronic supplementary material, S1) and not to sampling limitation. In the following year, we repeated the study and got essentially the same results (A = 64, P = 31, I = 268 visit links, M = 104 (i.e. additional pollen links), F = 1612).
(ii). Seed-dispersal networks
We constructed seed-dispersal/frugivory interaction matrices for the two sites (figure 1 and electronic supplementary material, S4). Seventeen and 22–25 frugivorous birds (± three mammals) consumed fruit from 16 and 25 plant species, I was 141 and 131–156, and C was 52 per cent and 24 to 25 per cent at HR and NC, respectively (pooled data for all years; table 1). A sample-accumulation analysis of the interaction records showed that we had robustly estimated the number of actual interactions occurring and the interaction matrix (electronic supplementary material, S2).
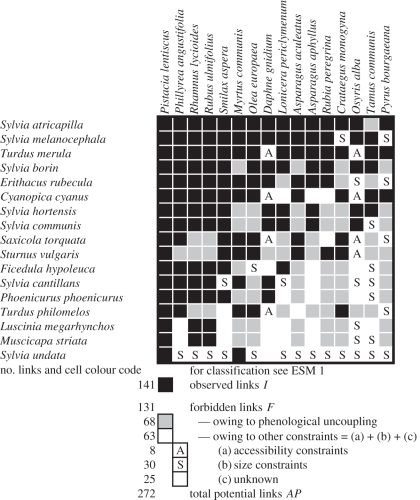
Figure 1.
On of the three studied interaction matrices: the seed-dispersal matrix from HR, showing all observed links (black cells) and all species combinations, which are phenologically uncoupled (grey cells). The link pattern is nested with seed dispersers listed in rows from top to bottom according to descending linkage level L, i.e. number of links per species; and plants are listed in columns from left to right also according to descending L. Cells with ‘S’ and ‘A’ indicate forbidden links owing to size mismatches and accessibility limitations, respectively. Given the robust sampling of these interaction matrices (electronic supplementary material, S2), blank cells indicate unobserved links attributable to other types of (unknown) constraints.
(b). Forbidden links
(i). Pollination network
A total of 1507 or 80 per cent of all possible links (i.e. of AP) in the combined visit–pollen matrix remained unobserved. The fact that the cumulative sampling analysis revealed a robust characterization of the existing interactions (electronic supplementary material, S2) suggested that these links could be regarded as forbidden (table 1 and electronic supplementary material, S3). Reasons for forbidden links are various constraints in action, especially phenological uncoupling. That alone explained 530 or 28 per cent of all possible links (table 1). Most phenological uncoupling took place in the lower right corner of the interaction matrix (i.e. in the link space between short-phenophase specialists; electronic supplementary material, S3). However, half of all unobserved links (80 − 28% = 52%) were forbidden because of constraints other than phenological uncoupling.
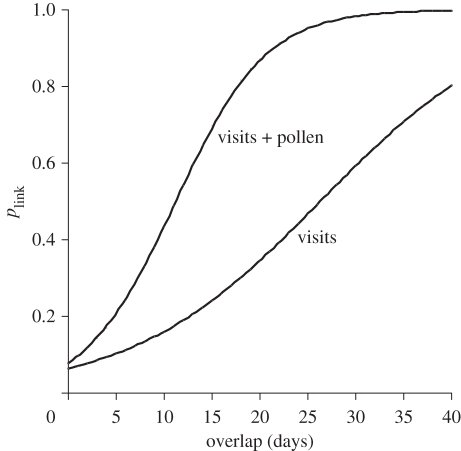
Here, we treated linkage constraints as all-or-none phenomena. However, linkage was probably most often partially constrained. This was certainly the case for phenological uncoupling. We calculated the number of overlapping days between the phenophases of any species pair of plant and pollinator within the study season and related this to the number of times a link was noted (i.e. we got an estimate of the link probability plink for a given overlap; figure 2). Increased phenological overlap between two species increased plink. Thus, links remained unobserved because of too weak a coupling or overlap of phenophases (e.g. 18 per cent of all species pairs had only a phenological overlap of 1 day and 43 per cent an overlap of 5 days or less). Extensive overlaps were needed to get high probabilities (e.g. plink ≥ 0.5 required an overlap of at least 27 and 11 days based on visit and visit–pollen data, respectively). Thus, especially the use of both visits and pollen as link currency increased plink considerably.
Figure 2.
Increase in probability p of observing a link with increasing phenophase overlap between a species pair in the pollination network. p is the fraction of species pairs with similar overlap in which a link is observed. Curves represent logistic regression models: visit data (y = exp(−2.68 + 0.102x)/(1 + exp(−2.687 + 0.102x)), Akaike information criterion = 237***; visit and pollen data (y = exp(−2.456 + 0.219x)/(1 + exp(−2.456 + 0.219x)), Akaike information criterion = 226***.
(ii). Seed-dispersal networks
Out of all possible links, 131 (48%, HR) and 419–469 (75–76%, NC) remained unobserved and were regarded as forbidden (table 1, figure 1 and electronic supplementary material, S4), given the robust estimation of interactions occurring in these communities (electronic supplementary material, S2). Phenological uncoupling explained 68 (25%, HR) and 135 (22–25%, NC) of all unobserved links. However, 23 per cent at HR and as much as 52–53% at NC were forbidden because of constraints other than complete phenological uncoupling. Thirty (HR) and 37 (NC) unobserved links were forbidden because of size mismatch between gape width and fruit diameter. Eight (HR) and 44–56 (NC) unobserved links were assumed forbidden because of accessibility limitations to the frugivores. The latter high figure at NC was due to the large-bodied Columba palumbus and Corvus corone, and the three mammals. They had problems in getting access to the fruit of plant species whose infructescence structure requires perching on thin branches for successful fruit handling (electronic supplementary material, S4).
In the seed-dispersal networks, increased phenological overlap between two species also increased the chance plink of observing a link (electronic supplementary material, S5). However, extensive overlaps were needed to get a high plink (e.g. plink ≥ 0.5 required an overlap of at least eight (HR) and 40 weeks (NC)). That we did observe phenological uncoupling in these seed-dispersal networks of ‘perennial’ species (birds, mammals) at all was because several bird species were migrants. The mammals in the network (Vulpes vulpes, Martes foina and Meles meles), however, were resident all year round.
4. Discussion
(a). Studies of missing links
Often, adjacency or presence/absence matrices are used to describe complex ecological networks, and frequently they turn out to be sparse in links. Given robust sampling, a large fraction of unobserved links is forbidden [3], which is best explained by linkage constraints that limit their occurrence. Two elements of inference are required in the analysis of unobserved interactions in plant–animal networks: first, an analysis of sampling robustness (i.e. to detect links between species of a low encounter rate); and second, natural history information on the participant species, which allows us to estimate the strength of linkage constraints. We predict that large fractions of unobserved links in ecological networks are, in fact, structural zeros, representing linkage constraints. An analysis of both expected and unexpected observed and unobserved links can therefore shed light on the evolution of complex networks.
(b). Observed and missing links in mutualistic networks
Our analysis of missing links followed procedures analogous to species-diversity sampling [32], using interaction accumulation curves ([2]; see also [25]). More sampling reduced the proportion of missing links. This is achieved by sampling full seasons, which reduced the proportion of missing links considerably (electronic supplementary material, S2). In addition, the use of an extra link currency (e.g. pollen) detected new links (C increased from 15 to 20%). Pollen data have previously been used in the construction of pollination networks. For Bosch et al. [22], pooling visit and pollen data increased C from 15 to 22 per cent, a result almost identical to ours. No additional link currencies exist for the HR and NC datasets or for any other plant–frugivore networks, but similar patterns can be expected. For instance, using focal observations to document infrequent visitation by rare frugivore species to rare fruiting plant species, or fruit-use interactions that naturally occur with extremely low frequency (e.g. involving toxic fruits) could be complemented with indirect sampling methods (e.g. camera traps or analyses of faecal samples). These additional links detected through pollen load and faecal analysis might be detected through observation alone, but this would require a larger study area and more field assistance.
Links between super-rare species in a network may be extremely difficult to detect because of their low species-encounter rate in the field. According to our classification (electronic supplementary material, S1), these links are missing. However, MacArthur [33] regarded very low abundance as a linkage constraint in itself because of the low chances of encounter. We may take this a step further. First, constraints are operating at the level of both individual and population. Two individuals cannot interact if constrained by one or more traits (e.g. a too-long corolla tube and a too-short tongue). A constraint is in full action between a plant population and a butterfly population if the range of the floral corolla tube length is completely above the range of the tongue length of the butterfly. The range of these traits is dependent upon species abundance: if species abundance decreases, the range of the trait becomes narrower. An extinction vortex may even run, when a species becomes rarer, gets a narrower range of its constraining traits, becomes more uncoupled to its mutualists, gets lower fitness, becomes even rarer, etc.
(c). Forbidden links
Forbidden links remain unobserved irrespective of sampling effort (i.e. they are a fact in natural communities). The cause is a battery of constraints, and a quantitatively important one is phenological uncoupling (e.g. [6,34]). In the mutualistic networks, 48 to 76 per cent of all links were forbidden and 22 to 25 per cent were so because of phenological uncoupling.
Temporal ecology has a long history. Elton [15] devoted a chapter in his Animal ecology to ‘Time and animal communities’. He wrote: ‘Many of the animals in a community never meet owing to the fact that they become active at different times’ (p. 83); that is, they are phenologically uncoupled. Today, phenological uncoupling is receiving much attention in relation to global climate change (e.g. [35–40]). Species respond differently in their onset and length of phenophase to climatic change [36,41]. Phenophase displacement among species may result in profound network changes. Thus, phenophase data will become baseline information, valuable to future studies estimating climate-driven changes on network structure, especially in the Arctic, where climate changes are most profound (e.g. [42]). However, the importance of phenological uncoupling varies with the length of season and latitude. The percentage of unobserved links explained by phenological uncoupling may range from 28 per cent in our Greenland pollination network to almost nil in networks where one or both of the interacting communities are perennial, such as in tropical domatia ant–plant networks [43], where mutualists are intimately linked to each other for extended periods. Phenological uncoupling can be important in habitats where organisms intrinsically have short phenophases, such as oligolectic bees with short flying periods, long-distance migratory frugivorous birds with short stop-over periods, etc.
Other constraints relate to reward, size and morphology; for example, butterflies paying no visits to nectarless ‘pollen flowers’, such as those of Papaver radicatum, or Diptera and small Hymenoptera unable to gain access to closed flowers, such as those of Pedicularis [11]. In our pollination network, only 3 per cent of all potential links may be forbidden because of these constraints (unpublished data), but as many as 14 to 15 per cent of all potential links in the seed-dispersal networks were due to size and accessibility constraints. Size constraints are also important in Mediterranean pollination networks [12]. However, we want to underline that a constraint is a property of the link, not of the involved species. A link may be constrained in one season, but not in the next; for example, the midge Limnophyes sp. was phenologically uncoupled with the plant Stellaria longipes in our study year, but in the subsequent year they were linked, or constrained, at HR but not at NC (e.g. between the bird Erithacus rubecula and the fruit tree Crataegus monogyna; figure 1 and electronic supplementary material, S4).
Phenological uncoupling is, like most constraints, not an all-or-none effect. We demonstrated that many unobserved links in the pollination network might be explained by a too-narrow phenological overlap between species (43% of all possible links had only an overlap of 5 days or less), resulting in low probabilities of interspecific encounter. Substantially larger overlaps were needed to make links more likely, although this might be due to other linkage constraints acting in concert with phenological uncoupling. Links may be weakly constrained either because of a weak expression (e.g. low concentration of toxins in a fruit) or because only parts of the interacting populations interact because of a partial range overlap of constraining traits; for example, a weak interaction between the most long-tongued individuals of a hawkmoth and orchid individuals with the shortest floral spur (e.g. [7]).
In the pollination network, linkage level and abundance are correlated [11]. Thus, common and generalist species seem to interact unconstrained; rare and specialized species are, first and foremost, constrained in their linkage by phenological uncoupling and low encounter rate; whereas species of intermediate abundance, phenophase and linkage level seem primarily to be limited by other linkage constraints (electronic supplementary material, S3). The situation with the plant–frugivore networks is analogous [6], with linkage level and local abundance (determined independently from the actual interaction frequency) being at least marginally correlated (P. Jordano 1980–2004, unpublished data).
Unobserved links are a characteristic feature of all ecological networks. Some remain unobserved because of insufficient sampling, whereas others are forbidden and are unobserved because of constraints. The latter fraction needs natural history details to explain their absence. Focusing just on the realized interactions and treating unobserved interactions as the expected unique result of sampling bias misses important components needed in our understanding of how mutualisms coevolve within complex networks of interdependence among species.
Acknowledgements
We are grateful to B. Elberling for field assistance and Theodora Petanidou, Amparo Lázaro and Diego Vázquez for many constructive comments and critique of this paper. Our grant donors were the Danish Natural Sciences Research Council (J.M.O., Y.D., H.E.), the Carlsberg Foundation (C.R.), the Spanish Ministerio de Ciencia y Tecnología (J.B. and P.J.; grants REN2003-04774 and CGL2006-00373), Junta de Andalucía (P07-RNM02824), the European Heads of Research Councils and the European Science Foundation through an EURYI award (J.B.), and we are most grateful for their support.
References
- 1.Montoya J. M., Pimm S. L., Solé R. V. 2006. Ecological networks and their fragility. Nature 442, 259–264 10.1038/nature04927 (doi:10.1038/nature04927) [DOI] [PubMed] [Google Scholar]
- 2.Jordano P., Vázquez D., Bascompte J. 2009. Redes complejas de interacciones planta-animal. In Ecología y evolución de las interacciones planta-animal: Conceptos y aplicaciones (eds Medel R., Aizen M., Zamora R.), pp. 17–41 Santiago, Chile: Editorial Universitaria [Google Scholar]
- 3.Jordano P., Bascompte J., Olesen J. M. 2003. Invariant properties in coevolutionary networks of plant–animal interactions. Ecol. Lett. 6, 69–81 10.1046/j.1461-0248.2003.00403.x (doi:10.1046/j.1461-0248.2003.00403.x) [DOI] [Google Scholar]
- 4.Genini J., Morellato L. P. C., Guimarães P. R., Jr, Olesen J. M. 2010. Cheaters in mutualism networks. Biol. Lett. 6, 494–497 10.1098/rsbl.2009.1021 (doi:10.1098/rsbl.2009.1021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snow B. K., Snow D. W. 1988. Birds and berries. Calton, UK: T. & A. D. Poyser [Google Scholar]
- 6.Jordano P. 1987. Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Am. Nat. 129, 657–677 [Google Scholar]
- 7.Nilsson L. A. 1988. The evolution of flowers with deep corolla tubes. Nature 334, 147–149 10.1038/334147a0 (doi:10.1038/334147a0) [DOI] [Google Scholar]
- 8.Gluckman P., Hanson M. 2006. Mismatch. Oxford, UK: Oxford University Press [Google Scholar]
- 9.Jordano P., Bacsompte J., Olesen J. M. 2006. The ecological consequences of complex topology and nested structure in pollination webs. In Plant–pollinator interactions: from specialization to generalization (eds Waser N. M., Ollerton J.), pp. 173–199 Chicago, IL: University of Chicago Press [Google Scholar]
- 10.Blüthgen N., Fründ J., Vázquez D. P., Menzel F. 2008. What do interaction network metrics tell us about specialization and biological traits? Ecology 89, 3387–3399 10.1890/07-2121.1 (doi:10.1890/07-2121.1) [DOI] [PubMed] [Google Scholar]
- 11.Olesen J. M., Bascompte J., Elberling H., Jordano P. 2008. Temporal dynamics of a pollination network. Ecology 89, 1573–1582 10.1890/07-0451.1 (doi:10.1890/07-0451.1) [DOI] [PubMed] [Google Scholar]
- 12.Stang M., Klinkhamer P. G. L., Waser N. M., Stang I., Meijden E. V. D. 2009. Size-specific interaction patterns and size matching in a plant–pollinator interaction web. Ann. Bot. 103, 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez D. P., Chacoff N. P., Cagnolo L. 2009. Evaluating multiple determinants of the structure of plant–animal mutualistic networks. Ecology 90, 2039–2046 10.1890/08-1837.1 (doi:10.1890/08-1837.1) [DOI] [PubMed] [Google Scholar]
- 14.Cushing D. H. 1975. Marine ecology and fisheries. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Elton C. 1927. Animal ecology. London, UK: Sidgwick & Jackson [Google Scholar]
- 16.Bloch E., Ritter M. 1977. Nonsynchronism and the obligation to its dialectics. New German Critique 11, 22–38 [Google Scholar]
- 17.Snow B. K., Snow D. W. 1972. Feeding niches of hummingbirds in a Trinidad valley. J. Anim. Ecol. 41, 471–485 [Google Scholar]
- 18.Stang M., Klinkhamer P. G. L., Meijden E. v. d. 2006. Size constraints and flower abundance determine the number of interactions in a plant–flower visitor web. Oikos 112, 111–121 10.1111/j.0030-1299.2006.14199.x (doi:10.1111/j.0030-1299.2006.14199.x) [DOI] [Google Scholar]
- 19.Welch C. A., Keay J., Kendall K. C., Robbins C. T. 1997. Constraints on frugivory by bears. Ecology 78, 1105–1109 10.1890/0012-9658(1997)078[1105:COFBB]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[1105:COFBB]2.0.CO;2) [DOI] [Google Scholar]
- 20.Santamaría L., Rodríguez-Gironés M. A. 2007. Linkage rules for plant–pollinator networks: trait complementarity or exploitation barriers? PLoS Biol. 5, e31. 10.1371/journal.pbio.0050031 (doi:10.1371/journal.pbio.0050031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallimanis A. S., Petanidou T., Tzanopoulos J., Pantis J. D., Sgardelis S. P. 2009. Do plant–pollinator interaction networks result from stochastic processes? Ecol. Model. 220, 684–693 10.1016/j.ecolmodel.2008.11.008 (doi:10.1016/j.ecolmodel.2008.11.008) [DOI] [Google Scholar]
- 22.Bosch J., Martin A. G., Anselm R., Navarro D. 2009. Plant–pollinator networks: adding the pollinator's perspective. Ecol. Lett. 12, 409–419 10.1111/j.1461-0248.2009.01296.x (doi:10.1111/j.1461-0248.2009.01296.x) [DOI] [PubMed] [Google Scholar]
- 23.Doyle A. C. 1893. The adventure of the ‘Gloria Scott’. Strand Mag. 5, 395–406 [Google Scholar]
- 24.Blüthgen N. 2010. Why network analysis is often disconnected from community ecology: a critique and an ecologist's guide. Basic Appl. Ecol. 11, 185–195 10.1016/j.baae.2010.01.001 (doi:10.1016/j.baae.2010.01.001) [DOI] [Google Scholar]
- 25.Nielsen A., Bascompte J. 2007. Ecological networks, nestedness and sampling effort. J. Ecol. 95, 1134–1141 10.1111/j.1365-2745.2007.01271.x (doi:10.1111/j.1365-2745.2007.01271.x) [DOI] [Google Scholar]
- 26.Jordano P. 1988. Diet, fruit choice and variation in body condition of frugivorous warblers in Mediterranean scrubland. Ardea 76, 193–209 [Google Scholar]
- 27.Jordano P., Schupp E. 2000. Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecol. Monogr. 70, 591–615 [Google Scholar]
- 28.Olesen J. M., Warncke E. 1989. Predation and transfer of pollen in a population of Saxifraga hirculus L. Holarctic Ecol. 12, 87–95 [Google Scholar]
- 29.Jordano P. 1985. El ciclo annual de los paseriformes frugívores en el matorral mediterráneo del sur de España: importancia de su invernada y variaciones interanuales. Ardeola 32, 69–94 [Google Scholar]
- 30.García-Castaño J. L. Consecuencias demográficas de la dispersión de semillas por aves y mamíferos frugívoros en la vegetación mediterránea de montaña. 2001. PhD dissertation, Universidad de Sevilla, Seville, Spain. [Google Scholar]
- 31.Carnicer J., Jordano P., Melián C. J. 2009. The temporal dynamics of resource use by frugivorous birds: a network approach. Ecology 90, 1958–1970 10.1890/07-1939.1 (doi:10.1890/07-1939.1) [DOI] [PubMed] [Google Scholar]
- 32.Gotelli N., Colwell R. K. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391 [Google Scholar]
- 33.MacArthur R. H. 1972. Geographical ecology: patterns in the distribution of species. Princeton, NJ: Princeton University Press [Google Scholar]
- 34.Vázquez D. P. 2005. Degree distribution in plant–animal mutualistic networks: forbidden links or random interactions? Oikos 108, 421–426 10.1111/j.0030-1299.2005.13619.x (doi:10.1111/j.0030-1299.2005.13619.x) [DOI] [Google Scholar]
- 35.Inouye D. W., Saavedra F., Lee-Wang W. 2003. Environmental influences on the phenology and abundance of flowering by Androsace septentrionalis (Primulaceae). Am. J. Bot. 90, 905–910 10.3732/ajb.90.6.905 (doi:10.3732/ajb.90.6.905) [DOI] [PubMed] [Google Scholar]
- 36.Høye T. T., Post E., Meltofte H., Schmidt N. M., Forchhammer M. C. 2007. Rapid advancement of spring in the high Arctic. Curr. Biol. 17, R449–R451 10.1016/j.cub.2007.04.047 (doi:10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- 37.Memmott J., Craze P. G., Waser N. W., Price M. V. 2007. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717 10.1111/j.1461-0248.2007.01061.x (doi:10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- 38.Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 10.1111/j.1461-0248.2008.01250.x (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 39.Hegland S. J., Nielsen A., Lázaro A., Bjerknes A.-L., Totland Ø. 2009. How does climate warming affect plant–pollinator interactions? Ecol. Lett. 12, 184–195 10.1111/j.1461-0248.2008.01269.x (doi:10.1111/j.1461-0248.2008.01269.x) [DOI] [PubMed] [Google Scholar]
- 40.Woodward G., Perkins D. M., Brown L. E. 2010. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Phil. Trans. R. Soc. B 365, 2093–2106 10.1098/rstb.2010.0055 (doi:10.1098/rstb.2010.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Körner C., Basler D. 2010. Phenology under global warming. Science 327, 1461. 10.1126/science.1186473 (doi:10.1126/science.1186473) [DOI] [PubMed] [Google Scholar]
- 42.Witze A. 2008. Losing Greenland. Nature 452, 798–802 10.1038/452798a (doi:10.1038/452798a) [DOI] [PubMed] [Google Scholar]
- 43.Fonseca C. R., Ganade G. 1996. Asymmetries, compartments and null interactions in an Amazonian ant-plant community. J. Anim. Ecol. 65, 339–347 [Google Scholar]