Abstract
Natural selection should strongly favour hosts that can protect themselves against parasites. Most studies on animals so far have focused on resistance, a series of mechanisms through which hosts prevent infection, reduce parasite growth or clear infection. However, animals may instead evolve tolerance, a defence mechanism by which hosts do not reduce parasite infection or growth, but instead alleviate the negative fitness consequences of such infection and growth. Here, we studied genetic variation in resistance and tolerance in the monarch butterfly (Danaus plexippus) to its naturally occurring protozoan parasite, Ophryocystis elektroscirrha. We exposed 560 monarch larvae of 19 different family lines to one of five different parasite inoculation doses (0, 1, 5, 10 and 100 infective spores) to create a range of parasite loads in infected butterflies. We then used two proxies of host fitness (adult lifespan and body mass) to quantify: (i) qualitative resistance (the ability to prevent infection; also known as avoidance or anti-infection resistance); (ii) quantitative resistance (the ability to limit parasite growth upon infection; also known as control or anti-growth resistance); and (iii) tolerance (the ability to maintain fitness with increasing parasite infection intensity). We found significant differences among host families in qualitative and quantitative resistance, indicating genetic variation in resistance. However, we found no genetic variation in tolerance. This may indicate that all butterflies in our studied population have evolved maximum tolerance, as predicted by some theoretical models.
Keywords: host–parasite interactions, resistance, tolerance, monarch butterfly, Ophryocystis elektroscirrha
1. Introduction
All organisms serve as hosts to one or more parasite species, and parasites can have large detrimental effects on host fitness [1]. In response, hosts have evolved many lines of behavioural, morphological and physiological defence that reduce the risk of infection or minimize parasite-induced fitness losses [2]. Hosts can avoid encountering the parasite [3], ingest compounds with anti-parasitic properties [4], mount an immune response [5] and reduce or compensate for the parasite-induced fitness loss [6]. These protective mechanisms have generally been categorized into two types of defence strategy: resistance and tolerance. Resistance includes mechanisms that prevent infection, reduce infection, or limit parasite growth upon infection, whereas tolerance refers to the alleviation of fitness loss owing to infection without reducing such infection [7].
Although these two defence strategies have the same function from the individual host's point of view—the preservation of fitness—their distinction is crucial as they can have different consequences for the ecological and evolutionary dynamics of host–parasite interactions [7–12]. The critical difference between resistance and tolerance is that resistance directly reduces parasite fitness, whereas tolerance does not. As a result, theoretical models have suggested that hosts may maintain genetic polymorphisms in resistance, but not in tolerance [8,9,11]. This is because the evolution of resistance should result in a negative epidemiological feedback through which a higher frequency of resistant hosts will reduce parasite transmission and prevalence to below the level at which resistant hosts are selected for (assuming that resistance is costly); once susceptible hosts increase in frequency again, parasite transmission and prevalence will increase, resulting in selection for resistant hosts once more. In contrast, with tolerance the epidemiological feedback is positive: when the frequency of tolerant hosts increases in the population, parasite transmission and prevalence increase, causing further selection for tolerant hosts.
Plant researchers have long considered the relative roles of resistance and tolerance to biotic and abiotic threats [13]. To date, numerous empirical studies have quantified the level of genetic variation in plant resistance and tolerance to herbivores (e.g. [14–17]; reviewed in [18,19]), herbicides [20,21] or parasites (e.g. [22–24]). In these studies, resistance is characterized as the plant's ability to decrease the intensity of damage or infection rate/intensity and tolerance as the slope of a reaction norm of fitness across a gradient of increasing damage/infection rate/infection intensity. In terms of parasitism, highly resistant plant genotypes then have lower infection rates or parasite burdens compared with less resistant plant genotypes, while highly tolerant plant genotypes suffer smaller reductions in fitness with increases in parasite infection rates or intensity compared with less tolerant plant genotypes.
The merit of these studies is not only that they have emphasized that organisms can use tolerance mechanisms instead of resistance mechanisms to defend themselves against parasites, but also that they have shown that genetic variation in tolerance is common [14,15,17,22,23]. Because such polymorphism contradicts simple theoretical predictions (but see [25]), many researchers have explored mechanisms that could maintain such variation, including fitness costs and trade-offs with resistance mechanisms [14,17,22,23]. More recently, theoretical models have also indicated that variation in tolerance may actually be expected to evolve when tolerance mechanisms act on maintaining host fecundity rather than host survival [25]. The reason for this difference is that when tolerance allows hosts to maintain their survival despite heavy parasite infection, parasite fitness can be enhanced by increasing the period over which transmission can occur. This leads to a positive epidemiological feedback, resulting in a fixation of tolerance. In contrast, when hosts tolerate infection by maintaining their fecundity instead, the effect on parasites may be neutral, and variation in tolerance could evolve [25].
A better understanding of defence mechanisms against pathogens in animals is of both fundamental and medical importance [26]. However, despite the existence of the straightforward statistical framework to test for tolerance, there are still few empirical studies of the role of tolerance in response to infectious diseases in animals [26–29]. In addition, tolerance studies in animals have so far focused on either non-natural host–parasite associations [30–32] or wild-caught animals [33], for which estimates of tolerance can be biased by random environmental factors affecting both host fitness and infection [28]. Despite these limitations, these studies have clearly demonstrated the existence of tolerance in animals. For example, experimental infection of laboratory mice with the rodent malaria parasite Plasmodium chabaudi showed that the increase in disease severity with increasing parasite burdens varied among five mouse strains [31]. This study also found a negative relationship between resistance and tolerance, suggesting a trade-off between these two defence strategies. This finding is consistent with theoretical considerations ([14,22,34]; but see [35]) and also supported by a recent study that showed that moderately heterozygous dace were less resistant but more tolerant to infection than highly heterozygous or homozygous dace [33,36].
Here, we used the statistical framework developed by plant biologists [18,37] and recently applied to animal systems [31,33] to study genetic variation in resistance and tolerance in a natural host–parasite interaction: the monarch butterfly (Danaus plexippus) and its protozoan parasite Ophryocystis elektroscirrha. A recent study [38] suggested that monarch butterflies may vary in their tolerance to these parasites based on the finding that infected—but not uninfected—monarch families varied in adult lifespan. However, this study was not specifically designed to study tolerance, did not control parasite loads and did not use the statistical framework as used in the plant literature. Therefore, to rigorously test for variation in tolerance in this system, we used 19 family lines of the monarch butterfly obtained from a natural population, and exposed these to a range of inoculation doses of a natural isolate of the parasite O. elektroscirrha obtained from the same population. In this system, higher inoculation doses result in higher parasite loads [39], such that inoculation doses can be used to experimentally control the parasite loads that monarchs will experience. We specifically used different inoculation doses to create a gradient of environments [18,37] across which proxies of host fitness (lifespan and body mass) could be measured. Experimentally manipulated levels of parasite load are more relevant than using uncontrolled variation in parasite load following a single inoculation dose or following infection with different parasite genotypes, because such variation may be caused by variation in host resistance or specific host–parasite genetic interactions (e.g. [38]), violating the independence of resistance and tolerance measures. To better understand the maintenance of genetic variation in defence mechanisms, we also studied fitness costs of defence and potential trade-offs between the different defence mechanisms.
2. Material and methods
(a). Study system
Monarch butterflies (D. plexippus) occur worldwide and are dependent on milkweeds as their larval host plants [40]. Monarchs are commonly infected with the apicomplexan protozoan parasite O. elektroscirrha [41,42]. Infection with this parasite occurs when caterpillars ingest parasite spores scattered onto eggs or host plant leaves by infected female monarchs during oviposition. In the larval midgut, spores release sporozoites that pass through the larval gut to invade the hypodermal tissues [43]. The parasites then undergo asexual and sexual replication, giving rise to a large number of spores on the outside of the body of the emerging butterfly [39]. Parasites do not continue to replicate on adults, and spores must be ingested by larvae to cause new infections. Previous work has shown that O. elektroscirrha strongly reduces monarch fitness, with infected monarchs having reduced pre-adult survival, adult lifespan, mating success and lifetime fecundity [38,39,44–47].
Monarchs used here were the laboratory-reared grand-progeny of wild female monarchs caught in Pismo Beach, CA, USA (35°8′ N, 120°38′ W) in December 2008. They were reared in the laboratory using cuttings of greenhouse-raised tropical milkweed, Asclepias curassavica. Newly emerged uninfected adults were selected to produce 19 non-inbred F2 family lines following previously established procedures [38,39,45–47].
The parasite clone used was derived from an infected butterfly collected in Pismo Beach (CA, USA) in 2005. The use of a single parasite clone avoids potential parasite genotype main effects and genetic interactions with host genotypes that would lead to variation in host fitness [38].
(b). Experimental design and procedures
Caterpillars were obtained by allowing female butterflies to oviposit on greenhouse-grown A. curassavica plants. Two days after hatching, when larvae had reached the second instar, they were transferred to individual 10 cm Petri dishes for parasite inoculation. These dishes contained moist filter paper and one 0.8 cm diameter leaf disc of A. curassavica on which we had deposited parasite spores manually using a drawn-out glass capillary tube; uninfected control larvae received leaf discs without spores. For each monarch family, we exposed caterpillars to each of the following inoculation doses: 0, 1, 5, 10 and 100 parasite spores. We used five replicate larvae per family per dose, except in the case of the one-spore dose, where we used 5–10 larvae; more replicates were used for the one-spore dose treatment to more accurately estimate the low proportions of monarchs that become infected when given this dose [39]. Overall, the experiment consisted of 25–30 caterpillars per family and 560 caterpillars in total.
Once larvae had completely eaten their milkweed leaf disc—and hence ingested the full inoculum of parasites—they were transferred to 1.34 l plastic tubes and reared individually with cuttings of A. curassavica (greenhouse-grown and parasite-free) at 26°C and a 16L : 8D light cycle. The containers were checked daily and larvae provided with fresh milkweed cuttings as needed until pupation. Once monarchs had been in the pupal stage for 6 days (an average of 3 days before adult emergence), they were transferred to a second laboratory, maintained at the same temperature and light cycle. This was done to avoid risk of parasite contamination of the larval rearing laboratory by emerging infected adults. After eclosion, adult monarchs were sexed, weighed, transferred to individual glassine envelopes held in a 14°C incubator and checked daily to record the date of host death. We then measured lifespan as the difference in days between the day of emergence and the day of death. This measure provides a combined index of adult monarch lifespan and starvation resistance, which can be crucially important for monarch survival during periods of food limitation such as occur during the overwintering phase [48,49]. Following host death, parasite spore load was determined by vortexing monarch bodies to shake off spores and counting these spores using a haemocytometer [39,46].
(c). Measurements of host resistance and tolerance
Two types of host resistance were recorded, following Gandon & Michalakis [50]. First, we measured qualitative resistance (also referred to as avoidance or anti-infection resistance) as the proportion of individuals that remain uninfected upon parasite exposure. Thus, qualitatively resistant monarch families are those that display low levels of infection. We specifically defined qualitative resistance for a family as the intercept of the regression line that described the relationship between the proportion of individuals that remained uninfected and inoculation dose for that family.
Second, quantitative resistance (also referred to as control or anti-growth resistance) was determined by measuring the parasite spore load of adult butterflies that became infected; this measure corresponds to the infection intensity (or parasite burden) and is positively related to the level of within-host parasite replication for a given inoculation dose. Quantitatively resistant monarch families are those that are efficient at reducing parasite growth, and hence suffer low parasite spore load. Specifically, we defined quantitative resistance for a family as the intercept of the regression line that described the relationship between parasite spore load and inoculation dose for that family.
Tolerance is often characterized by the slope of a regression of host fitness against parasite burden and/or level of parasite exposure [22,24,31,33]. A slope of zero indicates complete tolerance, whereas steep negative slopes indicate low levels of tolerance. We used adult monarch lifespan and mass as proxies for host fitness: both traits are important components of monarch fitness (e.g. [47,51]) and both are reduced by infection with O. elektroscirrha [38,39,44–47]. Moreover, both measures are important for understanding parasite fitness, as O. elektroscirrha is mostly transmitted from infected mothers to offspring. Monarchs with higher body mass live longer, and longer lifespans allow monarchs to obtain a higher probability of mating, as well as a higher number of oviposition opportunities. Since parasites are transmitted during oviposition, a higher mating probability and longer monarch lifespan increase parasite fitness [46].
For each family we analysed the slopes of the regression lines of these traits versus inoculation dose and parasite spore load. Accordingly, more tolerant families have less steep slopes and are able to maintain higher lifespan/mass with increasing inoculation dose/parasite spore load. We used both inoculation dose and spore load as explanatory variables to increase the power of detection of variation in tolerance. Although the best way to measure variation in tolerance would be to fully control a range of pre-determined parasite loads in infected monarchs, such control is not possible in this system. Instead, we used inoculation dose, as this measure can be fully controlled, and also because inoculation dose is strongly correlated with final parasite loads in this system [39]. However, the use of inoculation dose may confound the measurements of tolerance and resistance if monarch families vary in the slopes of their relationships between parasite spore load and inoculation dose. In that case, variation in the slopes between monarch fitness and inoculation dose may arise from the variation in slopes of the relationships between parasite load and inoculation dose, instead of variation in the slopes of the relationships between monarch fitness and parasite load. We therefore also used parasite spore load as an explanatory variable in the analyses of tolerance: although spore loads are not fully controlled, they do provide variation in parasite intensity across which host fitness can be measured.
(d). Statistical analysis
All analyses were carried out in R v. 2.7.1. Logistic regression by generalized linear mixed models (GLMM; binomial errors, logit link, using the lme4 package) was used to investigate the effect of inoculation dose, monarch family and sex on the probability of infection. Linear mixed models were used to analyse the effect of inoculation dose, monarch family and sex on parasite spore load, and to assess the effect of inoculation dose/parasite spore load, monarch family and sex on monarch adult lifespan and mass. A significant interaction between monarch family and inoculation dose/spore load indicates genetic variation in tolerance [22,31,33]. We also included the quadratic term of inoculation dose/parasite spore load to test for a nonlinear relationship between inoculation dose/parasite spore load and host fitness (longevity or mass) [31,33,34]. Uninfected monarch butterflies (infection intensity equal to zero) were included in the analysis to account for the fitness of each monarch family in the absence of damage, and hence distinguish between tolerance and general vigour [18]. The conclusions drawn from statistical analyses of genetic variation in disease tolerance were not changed when conducted on exposed individuals only or on infected individuals only (results not shown).
Parasite spore loads and inoculation dose were log10-transformed and models were checked for homogeneity of variance by using Fligner–Killeen tests [52]. In these analyses, inoculation dose and parasite spore load were treated as a continuous variable, and host family and sex were treated as categorical explanatory variables. Full models included inoculation dose, host family and monarch sex as explanatory variables, and interactions between them.
Minimal models were derived by removing model terms followed by model comparison. Only terms for which removal significantly (p < 0.05) reduced the explanatory power of the model were retained in the minimal model [52]. Analyses of resistance and tolerance with monarch family specified as fixed versus random effects yielded the same outcomes, and we report on analyses that considered monarch family as a random effect.
To study the costs associated with resistance, we used linear regression models to test for significant association between qualitative/quantitative resistance and host fitness (lifespan and mass) in uninfected controls. Similarly, we used linear regression models to examine the relationship between qualitative and quantitative resistance. Because we found no evidence for genetic variation on tolerance in this study, we were not able to analyse fitness costs of tolerance.
3. Results
A total of 91 out of 95 (96%) uninfected control monarchs and 445 out of 465 (96%) inoculated monarchs (with doses of 1, 5, 10 and 100 parasite spores) survived to the adult stage. All subsequent analyses are restricted to these surviving monarchs.
(a). Qualitative resistance
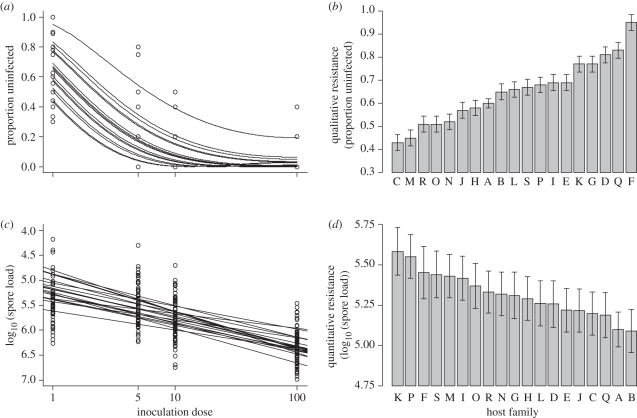
Of the 445 surviving monarchs that had been inoculated, a total of 301 (68%) became infected. Inoculation dose had a strong effect on the probability of infection: the proportion of monarchs that remained uninfected strongly decreased with increasing inoculation doses (χ2 = 145, d.f. = 1, p < 0.001; figure 1a). The intercepts of the relationships between the proportion of uninfected monarchs and inoculation dose significantly differed between monarch families, revealing genetic variation in qualitative resistance (χ2 = 32.5, d.f. = 18, p = 0.019; figure 1b). Continuous variation in qualitative resistance among the families was observed, indicating that there were no completely qualitatively resistant or completely susceptible families. We found no significant family × inoculation dose interaction (χ2 = 3.5, d.f. = 18, p = 0.24). Monarch sex did not affect the probability of infection (χ2 = 0.94, d.f. = 1, p = 0.33).
Figure 1.
Monarch resistance to O. elektroscirrha. (a) Effect of inoculation dose on the proportion of monarchs that remained uninfected for each monarch family line. Note that the lines shown represent least-squares regression lines for monarch families on the basis of arcsine-square-root-transformed proportions; intercepts, but not slopes, are significantly different on the basis of logistic regression (see §3). (b) Variation in qualitative resistance (expressed as the intercept ± s.e. of the regression lines in (a)) observed among the 19 host families, ranked from least to most qualitatively resistant. (c) Effect of inoculation dose on parasite spore load in animals that became infected for each monarch family. Note that the y-axis is inverted because lower parasite spore load indicates higher quantitative resistance. Lines represent regression lines fitted for each monarch family: intercepts, but not slopes, vary significantly among families. (d) Variation in quantitative resistance (expressed as the intercept ± s.e. of regression lines in (c)) observed among the 19 host families, ranked from least to most quantitatively resistant.
(b). Quantitative resistance
Higher inoculation doses resulted in greater parasite spore loads in monarchs that became infected (χ2 = 185, d.f. = 1, p < 0.01; figure 1c). The intercept of the relationship between spore load and inoculation dose varied by monarch family, indicating genetic variation in quantitative resistance (χ2 = 38, d.f. = 18, p = 0.012; figure 1d). There was no significant interaction between inoculation dose and host family (χ2 = 17, d.f. = 18, p > 0.05). Host sex also had no effect on parasite spore load (χ2 = 3, d.f. = 1, p > 0.05).
(c). Tolerance
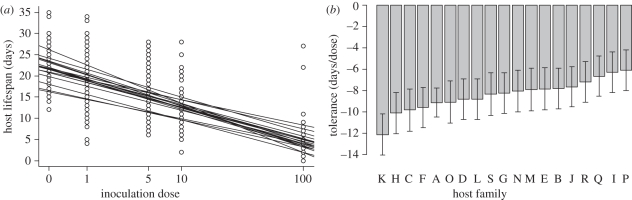
Inoculation dose had a strong effect on adult lifespan (table 1, figure 2a). Host lifespan significantly varied among monarch families (table 1, figure 2b), but there was no significant interaction between host family and inoculation dose (table 1). These results indicate that host families vary in general vigour [18], but not tolerance in terms of host lifespan. Host sex had no effect on host lifespan (table 1).
Table 1.
Analysis of genetic variation in disease tolerance using inoculation dose as a measure of infection intensity. The two-way interactions between monarch family and inoculation dose were not statistically significant, indicating no genetic variation in tolerance. Other non-significant two-way interactions are not represented. Significance (p < 0.05) is indicated by asterisks.
| χ2 | d.f. | p | |
|---|---|---|---|
| adult lifespan | |||
| inoculation dose | χ2 = 470 | d.f. = 1 | p < 0.001* |
| (inoculation dose)2 | χ2 = 0.02 | d.f. = 1 | p = 0.9 |
| monarch family | χ2 = 98 | d.f. = 18 | p = 0.02* |
| monarch sex | χ2 = 2 | d.f. = 1 | p = 0.16 |
| monarch family × inoculation dose | χ2 = 21.8 | d.f. = 18 | p = 0.24 |
| monarch family × (inoculation dose)2 | χ2 = 18.3 | d.f. = 18 | p = 0.44 |
| adult mass | |||
| inoculation dose | χ2 = 0.76 | d.f. = 1 | p = 0.38 |
| (inoculation dose)2 | χ2 = 0.3 | d.f. = 1 | p = 0.57 |
| monarch family | χ2 = 81 | d.f. = 18 | p = 0.035* |
| monarch sex | χ2 = 6.9 | d.f. = 1 | p = 0.008* |
| monarch family × inoculation dose | χ2 = 26.7 | d.f. = 18 | p = 0.08 |
| monarch family × (inoculation dose)2 | χ2 = 20.4 | d.f. = 18 | p = 0.31 |
Figure 2.
Monarch tolerance to O. elektroscirrha. (a) Effect of inoculation dose on host lifespan for each monarch family line. Lines show least-squares regressions fitted for the 19 host families; intercepts, but not slopes, vary significantly. (b) Tolerance (expressed as the slopes of regression lines in (a)) observed among the 19 host families; note that families do not vary significantly in their tolerance.
Inoculation dose did not affect adult mass (table 1), but adult mass varied among monarch families (table 1). There was no significant interaction between host family and inoculation dose (table 1), indicating an absence of genetic variation in disease tolerance in terms of body mass. Host sex had an effect on host mass, with males being larger on average than females (table 1; mean ± s.e. adult mass: male: 0.67 g ± 0.008, female: 0.64 g ± 0.007). There was no significant sex × family interaction.
Because parasite spore load was not significantly affected by an interaction between inoculation dose and monarch family (see §3b), the use of inoculation dose as a measure of parasite load in the analyses of tolerance was justified, because any variation in the slopes of the relationships between lifespan/body mass and inoculation dose could not have been accounted for by variation in the slopes of the relationships between spore load and inoculation dose. However, we still carried out a second analysis of tolerance on the basis of parasite spore load as measure of parasite intensity. This analysis yielded similar effects as the analysis using inoculation dose. Thus, monarchs lived for a much shorter time (but did not weigh less) with increasing parasite spore loads, and monarch families varied in the lifespan and mass they obtained for a given spore load, but there were no significant interactions between host family and spore load. These results again indicate a lack of genetic variation for tolerance in terms of host lifespan or body mass (see table 2).
Table 2.
Analysis of genetic variation in disease tolerance using parasite spore load as measure of infection intensity. The two-way interactions between monarch family and parasite spore load were not statistically significant, indicating no genetic variation in tolerance. Other non-significant two-way interactions are not represented. Significance (p < 0.05) is indicated by asterisks.
| χ2 | d.f. | p | |
|---|---|---|---|
| adult lifespan | |||
| spore load | χ2 = 81.52 | d.f. = 1 | p < 0.001* |
| (spore load)2 | χ2 = 155 | d.f. = 1 | p < 0.001* |
| monarch family | χ2 = 29.2 | d.f. = 18 | p = 0.039* |
| monarch sex | χ2 = 0.09 | d.f. = 1 | p = 0.77 |
| monarch family × spore load | χ2 = 27.9 | d.f. = 18 | p = 0.064 |
| monarch family × (spore load)2 | χ2 = 28.1 | d.f. = 18 | p = 0.056 |
| adult mass | |||
| spore load | χ2 = 3.37 | d.f. = 1 | p = 0.066 |
| (spore load)2 | χ2 = 1.7 | d.f. = 1 | p = 0.2 |
| monarch family | χ2 = 30 | d.f. = 18 | p = 0.04* |
| monarch sex | χ2 = 6.14 | d.f. = 1 | p = 0.01* |
| monarch family × spore load | χ2 = 26.6 | d.f. = 18 | p = 0.11 |
| monarch family × (spore load)2 | χ2 = 29 | d.f. = 18 | p = 0.059 |
(d). Costs of resistance
No costs of qualitative resistance were found in terms of host lifespan or mass: qualitatively resistant monarch families did not have shorter lifespan and were not smaller than less qualitatively resistant monarchs in the absence of parasites (lifespan: F1,17 = 0.1, p = 0.75; mass: F1,17 = 0.01, p = 0.9). Similarly, no costs of quantitative resistance were found in terms of host lifespan or mass (lifespan: F1,17 = 0.69, p = 0.4; mass: F1,17 = 2, p = 0.17). Finally, no association between qualitative and quantitative resistance was found (F1,17 = 2.1, p = 0.17). Given that we found no evidence for genetic variation in tolerance, we were not able to test for fitness costs of tolerance.
4. Discussion
Our results suggest that monarch butterflies vary genetically in resistance, but not tolerance, to their protozoan parasite O. elektroscirrha. Evidence for variation in qualitative resistance comes from the finding that the 19 monarch families used here varied in the proportion of animals that remained uninfected upon exposure to four inoculation doses of the parasite. Moreover, among animals that became infected, monarch families varied in the spore load that parasites produced, indicating genetic variation in quantitative resistance. However, we found no evidence that monarch families varied in the slopes of their relationships between two fitness proxies (adult lifespan and body mass) and inoculation dose or parasite spore load. This suggests that monarch families do not vary in the fitness reductions that they suffer with increasing parasite exposure and burden, and indicates an absence of genetic variation in tolerance.
Our finding of genetic variation in disease resistance confirms many empirical studies, as well as theoretical predictions. Genetic variation in qualitative and quantitative resistance has been reported in a wide range of plant–parasite and animal–parasite associations (e.g. [53–57]), and there are at least two explanations for the maintenance of such variation. First, theoretical models have shown that host genetic variation in resistance can be maintained by costs associated with resistance [8,58–60], and costs of resistance have been found in some studies (e.g. [23,61–63]). In the current study, we did not find evidence for such costs, as more resistant monarch families did not have lower lifespan and body mass in the absence of parasites. This may indicate that there are no costs of resistance [64,65] or that such costs can only be detected under more natural, stressful or competitive conditions (e.g. [61,66,67]). Alternatively, it is possible that resistance confers costs to other—unmeasured—fitness components, such as fecundity. A second explanation for the maintenance of variation in resistance is the coevolutionary arms race between hosts and parasites, which is expected to result in specific genetic interactions between host and parasite genotypes. Thus, hosts resistant to a particular parasite genotype may be susceptible to other parasite genotypes. De Roode & Altizer [38] demonstrated such host–parasite genetic interactions in the monarch–parasite system, providing a potential explanation for the observed genetic variation in host resistance in this study.
The finding that monarch families did not vary in tolerance supports the predictions of a number of theoretical models on the evolution of tolerance. These models have indicated that positive frequency-dependent selection through an ecological feedback with disease prevalence can drive tolerance to fixation within populations [8–11]. The reason is that if an allele confers a greater level of tolerance, it can be favoured by natural selection over other alleles; the allele will then spread through the host population and the parasite prevalence will concomitantly increase. As the parasite becomes more common in the environment, selection for host tolerance is boosted, and the positive feedback pushes the allele to fixation [8,9,11]. Thus, the observed lack of polymorphism in tolerance in our study population may indicate that all hosts have already evolved the maximum tolerance possible [68–70]. It is important to note here that not all theoretical models predict a lack of genetic variation in tolerance. For instance, Best et al. [25] showed that variation in tolerance can evolve when tolerance acts on maintaining host fecundity (‘sterility tolerance’) instead of survival (‘mortality tolerance’). This model showed that mortality tolerance—by increasing the period over which parasite transmission occurs—can enhance parasite fitness and result in a positive epidemiological feedback loop. In contrast, sterility tolerance was neutral with regard to parasite fitness, and resulted in the maintenance of genetic variation in tolerance. However, in the monarch–parasite system, host and parasite fecundity are much intertwined, because parasites are transmitted to monarch offspring during oviposition, and longer monarch lifespans allow for more oviposition events. Hence, the maintenance of both survival and fecundity would result in a concomitant increase in parasite fitness [46] and thereby drive tolerance towards fixation.
An alternative explanation for the observed lack of genetic variation in tolerance is that monarch butterflies have not in fact evolved tolerance mechanisms over the range of infection intensities that we studied. This may be because they already have a comprehensive arsenal of defence mechanisms that can render tolerance superfluous. For instance, the current study shows that monarchs possess both qualitative and quantitative resistance, and a recent study has also shown that monarchs have a behavioural defence mechanism through which they can preferentially lay their eggs on anti-parasitic host plants (T. Lefèvre, L. Oliver, M. D. Hunter & J. C. de Roode 2009, unpublished results). It is often suggested that organisms need not invest in multiple defence mechanisms at once [21,71,72], and a recent review has suggested that free-moving (as opposed to sessile) organisms may invest more heavily in defence mechanisms other than tolerance [29]. The reason for this is that sessile organisms cannot move away from parasite threats and are more dependent on dealing with unavoidable damage. For example, plants have evolved a greater capacity to regenerate tissues [73,74], while free-moving animals can use behavioural mechanisms to avoid infection or medicate themselves upon infection [75]. However, although monarchs may not possess tolerance against infection over the range of parasite loads that we studied, we think it is unlikely that they have no tolerance at all over the full range of infection intensities they may suffer in the wild. As such, we favour the explanation that tolerance against O. elektroscirrha has become fixed in this population of monarch butterflies.
Acknowledgements
We thank R. Rarick and D. Frey for their help with collecting butterflies, C. Lopez and R. Rarick for help with the experiments, and Emory's Invertebrate Host–Parasite journal club, S. Blanchet, M. Boots, J. Koella and L. Råberg for constructive comments on the manuscript. This work was supported by Emory University and a Singer-Polignac stipend to T.L.
References
- 1.Windsor D. A. 1998. Most of the species on Earth are parasites. Int. J. Parasitol. 28, 1939–1941 10.1016/S0020-7519(98)00153-2 (doi:10.1016/S0020-7519(98)00153-2) [DOI] [PubMed] [Google Scholar]
- 2.Combes C. 2001. Parasitism: the ecology and evolution of intimate interactions. Chicago, IL: University of Chicago Press [Google Scholar]
- 3.Moore J. 2002. Parasites and the behavior of animals. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Hart B. L. 2005. The evolution of herbal medicine: behavioural perspectives. Anim. Behav. 70, 975–989 10.1016/j.anbehav.2005.03.005 (doi:10.1016/j.anbehav.2005.03.005) [DOI] [Google Scholar]
- 5.Frank S. A. 2002. Immunology and evolution of infectious disease. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 6.Michalakis Y. 2009. Parasitism and the evolution of life-history traits. In Ecology and evolution of parasitism (eds Thomas F., Guégan J. F., Renaud F.). Oxford, UK: Oxford University Press [Google Scholar]
- 7.Rausher M. D. 2001. Co-evolution and plant resistance to natural enemies. Nature 411, 857–864 10.1038/35081193 (doi:10.1038/35081193) [DOI] [PubMed] [Google Scholar]
- 8.Boots M., Bowers R. G. 1999. Three mechanisms of host resistance to microparasites—avoidance, recovery and tolerance—show different evolutionary dynamics. J. Theor. Biol. 201, 13–23 10.1006/jtbi.1999.1009 (doi:10.1006/jtbi.1999.1009) [DOI] [PubMed] [Google Scholar]
- 9.Roy B. A., Kirchner J. W. 2000. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54, 51–63 [DOI] [PubMed] [Google Scholar]
- 10.Restif O., Koella J. C. 2003. Shared control of epidemiological traits in a coevolutionary model of host–parasite interactions. Am. Nat. 161, 827–836 10.1086/375171 (doi:10.1086/375171) [DOI] [PubMed] [Google Scholar]
- 11.Miller M. R., White A., Boots M. 2005. The evolution of host resistance: tolerance and control as distinct strategies. J. Theor. Biol. 236, 198–207 10.1016/j.jtbi.2005.03.005 (doi:10.1016/j.jtbi.2005.03.005) [DOI] [PubMed] [Google Scholar]
- 12.Miller M. R., White A., Boots M. 2006. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution 60, 945–956 [PubMed] [Google Scholar]
- 13.Painter R. H. 1951. Insect resistance in crop plants. New York, NY: Wiley [Google Scholar]
- 14.Fineblum W. L., Rausher M. D. 1995. Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 377, 517–520 10.1038/377517a0 (doi:10.1038/377517a0) [DOI] [Google Scholar]
- 15.Mauricio R., Rausher M. D., Burdick D. S. 1997. Variation in the defense strategies of plants: are resistance and tolerance mutually exclusive? Ecology 78, 1301–1311 10.1890/0012-9658(1997)078[1301:VITDSO]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[1301:VITDSO]2.0.CO;2) [DOI] [Google Scholar]
- 16.Agrawal A. A., Strauss S. Y., Stout M. J. 1999. Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution 53, 1093–1104 10.2307/2640814 (doi:10.2307/2640814) [DOI] [PubMed] [Google Scholar]
- 17.Tiffin P., Rausher M. D. 1999. Genetic constraints and selection acting on tolerance to herbivory in the common morning glory Ipomoea purpurea. Am. Nat. 154, 700–716 10.1086/303271 (doi:10.1086/303271) [DOI] [PubMed] [Google Scholar]
- 18.Stowe K. A., Marquis R. J., Hochwender C. G., Simms E. L. 2000. The evolutionary ecology of tolerance to consumer damage. Annu. Rev. Ecol. Syst. 31, 565–595 10.1146/annurev.ecolsys.31.1.565 (doi:10.1146/annurev.ecolsys.31.1.565) [DOI] [Google Scholar]
- 19.Núñez-Farfán J., Fornoni J., Valverde P. L. 2007. The evolution of resistance and tolerance to herbivores. Annu. Rev. Ecol. Syst. 38, 541–566 [Google Scholar]
- 20.Baucom R. S., Mauricio R. 2004. Fitness costs and benefits of novel herbicide tolerance in a noxious weed. Proc. Natl Acad. Sci. USA 101, 13 386–13 390 10.1073/pnas.0404306101 (doi:10.1073/pnas.0404306101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baucom R. S., Mauricio R. 2008. Constraints on the evolution of tolerance to herbicide in the common morning glory: resistance and tolerance are mutually exclusive. Evolution 62, 2842–2854 10.1111/j.1558-5646.2008.00514.x (doi:10.1111/j.1558-5646.2008.00514.x) [DOI] [PubMed] [Google Scholar]
- 22.Simms E. L., Triplett J. 1994. Costs and benefits of plant responses to disease-resistance and tolerance. Evolution 48, 1973–1985 10.2307/2410521 (doi:10.2307/2410521) [DOI] [PubMed] [Google Scholar]
- 23.Koskela T., Puustinen S., Salonen V., Mutikainen P. 2002. Resistance and tolerance in a host plant–holoparasitic plant interaction: genetic variation and costs. Evolution 56, 899–908 [DOI] [PubMed] [Google Scholar]
- 24.Kover P. X., Schaal B. A. 2002. Genetic variation for disease resistance and tolerance among Arabidopsis thaliana accessions. Proc. Natl Acad. Sci. USA 99, 11 270–11 274 10.1073/pnas.102288999 (doi:10.1073/pnas.102288999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best A., White A., Boots M. 2008. Maintenance of host variation in tolerance to pathogens and parasites. Proc. Natl Acad. Sci. USA 105, 20 786–20 791 10.1073/pnas.0809558105 (doi:10.1073/pnas.0809558105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider D. S., Ayres J. S. 2008. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 8, 889–895 10.1038/nri2432 (doi:10.1038/nri2432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read A. F., Graham A. L., Råberg L. 2008. Animal defenses against infectious agents: is damage control more important than pathogen control? PLoS Biol. 6, 2638–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Råberg L., Graham A. L., Read A. F. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49 10.1098/rstb.2008.0184 (doi:10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baucom R. S., De Roode J. C. In press Ecological immunology and tolerance in plants and animals. Funct. Ecol. (doi:10.1111/j.1365-2435.2010.01742.x) [Google Scholar]
- 30.Corby-Harris V., Habel K. E., Ali F. G., Promislow D. E. L. 2007. Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J. Evol. Biol. 20, 526–533 10.1111/j.1420-9101.2006.01267.x (doi:10.1111/j.1420-9101.2006.01267.x) [DOI] [PubMed] [Google Scholar]
- 31.Råberg L., Sim D., Read A. F. 2007. Disentangling genetic variation for resistance and tolerance to infectious disease in animals. Science 318, 318–320 [DOI] [PubMed] [Google Scholar]
- 32.Ayres J. S., Schneider D. S. 2008. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 6, 2764–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchet S., Rey O., Loot G. 2010. Evidence for host variation in parasite tolerance in a wild fish population. Evol. Ecol. 24, 1129–1139 10.1007/s10682-010-9353-x (doi:10.1007/s10682-010-9353-x) [DOI] [Google Scholar]
- 34.Tiffin P. 2000. Mechanisms of tolerance to herbivore damage: what do we know? Evol. Ecol. 14, 523–536 10.1023/A:1010881317261 (doi:10.1023/A:1010881317261) [DOI] [Google Scholar]
- 35.Restif O., Koella J. C. 2004. Concurrent evolution of resistance and tolerance to pathogens. Am. Nat. 164, E90–E102 10.1086/423713 (doi:10.1086/423713) [DOI] [PubMed] [Google Scholar]
- 36.Blanchet S., Rey O., Berthier P., Lek S., Loot G. 2009. Evidence of parasite-mediated disruptive selection on genetic diversity in a wild fish population. Mol. Ecol. 18, 1112–1123 10.1111/j.1365-294X.2009.04099.x (doi:10.1111/j.1365-294X.2009.04099.x) [DOI] [PubMed] [Google Scholar]
- 37.Simms E. L. 2000. Defining tolerance as a norm of reaction. Evol. Ecol. 14, 563–570 10.1023/A:1010956716539 (doi:10.1023/A:1010956716539) [DOI] [Google Scholar]
- 38.De Roode J. C., Altizer S. 2010. Host–parasite genetic interactions and virulence–transmission relationships in natural populations of monarch butterflies. Evolution 64, 502–514 10.1111/j.1558-5646.2009.00845.x (doi:10.1111/j.1558-5646.2009.00845.x) [DOI] [PubMed] [Google Scholar]
- 39.De Roode J. C., Gold L. R., Altizer S. 2007. Virulence determinants in a natural butterfly–parasite system. Parasitology 134, 657–668 10.1017/S0031182006002009 (doi:10.1017/S0031182006002009) [DOI] [PubMed] [Google Scholar]
- 40.Ackery P. R., Vane-Wright R. I. 1984. Milkweed butterflies: their cladistics and biology. Ithaca, NY: Cornell University Press [Google Scholar]
- 41.Leong K. L., Yoshimura M. A., Kaya H. K., Williams H. 1997. Instar susceptibility of the monarch butterfly (Danaus plexippus) to the neogregarine parasite, Ophryocystis elektroscirrha. J. Invertebr. Pathol. 69, 79–83 10.1006/jipa.1996.4634 (doi:10.1006/jipa.1996.4634) [DOI] [PubMed] [Google Scholar]
- 42.Altizer S. M., Oberhauser K. S., Brower L. P. 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol. Entomol. 25, 125–139 10.1046/j.1365-2311.2000.00246.x (doi:10.1046/j.1365-2311.2000.00246.x) [DOI] [Google Scholar]
- 43.McLaughlin R. E., Myers J. 1970. Ophryocystis elektroscirrha sp. n., a neogregarine pathogen of monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer. J. Protozool. 17, 300–305 [Google Scholar]
- 44.Altizer S. M., Oberhauser K. S. 1999. Effects of the protozoan parasite Ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). J. Invertebr. Pathol. 74, 76–88 10.1006/jipa.1999.4853 (doi:10.1006/jipa.1999.4853) [DOI] [PubMed] [Google Scholar]
- 45.De Roode J. C., Pedersen A. B., Hunter M. D., Altizer S. 2008. Host plant species affects virulence in monarch butterfly parasites. J. Anim. Ecol. 77, 120–126 10.1111/j.1365-2656.2007.01305.x (doi:10.1111/j.1365-2656.2007.01305.x) [DOI] [PubMed] [Google Scholar]
- 46.De Roode J. C., Yates A. J., Altizer S. 2008. Virulence–transmission trade-offs and population divergence in virulence in a naturally occuring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489–7494 10.1073/pnas.0710909105 (doi:10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Roode J. C., Chi J., Rarick R. M., Altizer S. 2009. Strength in numbers: high parasite burdens increase transmission of a protozoan parasite of monarch butterflies (Danaus plexippus). Oecologia 161, 67–75 [DOI] [PubMed] [Google Scholar]
- 48.Alonso-Mejia A., Rendon-Salinas E., Montesinos-Patino E., Brower L. P. 1997. Use of lipid reserves by monarch butterflies overwintering in Mexico: implications for conservation. Ecol. Appl. 7, 934–947 10.1890/1051-0761(1997)007[0934:UOLRBM]2.0.CO;2 (doi:10.1890/1051-0761(1997)007[0934:UOLRBM]2.0.CO;2) [DOI] [Google Scholar]
- 49.Brower L. P. 1999. Biological necessities for monarch butterfly overwintering in relation to the oyamel forest ecosystem in Mexico. In The 1997 North American Conf. on the Monarch Butterfly (eds Hoth J., et al.), pp. 11–28 Montreal, Canada: Commission for Environmental Cooperation [Google Scholar]
- 50.Gandon S., Michalakis Y. 2000. Evolution of parasite virulence against qualitative or quantitative host resistance. Proc. R. Soc. Lond. B 267, 985–990 10.1098/rspb.2000.1100 (doi:10.1098/rspb.2000.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberhauser K. S. 1997. Fecundity, lifespan and egg mass in butterflies: effects of male-derived nutrients and female size. Funct. Ecol. 11, 166–175 10.1046/j.1365-2435.1997.00074.x (doi:10.1046/j.1365-2435.1997.00074.x) [DOI] [Google Scholar]
- 52.Crawley M. J. 2002. Statistical computing: an introduction to data analysis using S-Plus. Chichester, UK: Wiley [Google Scholar]
- 53.Thompson J. N., Burdon J. J. 1992. Gene-for-gene coevolution between plants and parasites. Nature 360, 121–125 10.1038/360121a0 (doi:10.1038/360121a0) [DOI] [Google Scholar]
- 54.Carius H. J., Little T. J., Ebert D. 2001. Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145 [DOI] [PubMed] [Google Scholar]
- 55.Niaré O., et al. 2002. Genetic loci affecting resistance to human malaria parasites in a West African mosquito vector population. Science 298, 213–216 10.1126/science.1073420 (doi:10.1126/science.1073420) [DOI] [PubMed] [Google Scholar]
- 56.Lambrechts L., Fellous S., Koella J. C. 2006. Coevolutionary interactions between host and parasite genotypes. Trends Parasitol. 22, 12–16 10.1016/j.pt.2005.11.008 (doi:10.1016/j.pt.2005.11.008) [DOI] [PubMed] [Google Scholar]
- 57.Poland J. A., Balint-Kurti P. J., Wisser R. J., Pratt R. C., Nelson R. J. 2009. Shades of gray: the world of quantitative disease resistance. Trends Plant Sci. 14, 21–29 10.1016/j.tplants.2008.10.006 (doi:10.1016/j.tplants.2008.10.006) [DOI] [PubMed] [Google Scholar]
- 58.Haldane J. 1949. Disease and evolution. La Ricerca Sci. Suppl. 19, 68–76 [Google Scholar]
- 59.Antonovics J., Thrall P. H. 1994. Cost of resistance and the maintenance of genetic polymorphism in host–pathogen systems. Proc. R. Soc. Lond. B 257, 105–110 10.1098/rspb.1994.0101 (doi:10.1098/rspb.1994.0101) [DOI] [Google Scholar]
- 60.Frank S. A. 1994. Recognition and polymorphism in host–parasite genetics. Phil. Trans. R. Soc. Lond. B 346, 283–293 10.1098/rstb.1994.0145 (doi:10.1098/rstb.1994.0145) [DOI] [PubMed] [Google Scholar]
- 61.Kraaijeveld A. R., Godfray H. C. J. 1997. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280 [DOI] [PubMed] [Google Scholar]
- 62.Webster J. P., Woolhouse M. E. J. 1999. Cost of resistance: relationship between reduced fertility and increased resistance in a snail–schistosome host–parasite system. Proc. R. Soc. Lond. B 266, 391–396 10.1098/rspb.1999.0650 (doi:10.1098/rspb.1999.0650) [DOI] [Google Scholar]
- 63.Boots M., Begon M. 1993. Trade-offs with resistance to a granulosis virus in the Indian meal moth, examined by a laboratory evolution experiment. Funct. Ecol. 7, 528–534 10.2307/2390128 (doi:10.2307/2390128) [DOI] [Google Scholar]
- 64.Coustau C., Chevillon C., Ffrench-Constant R. 2000. Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol. Evol. 15, 378–383 10.1016/S0169-5347(00)01929-7 (doi:10.1016/S0169-5347(00)01929-7) [DOI] [PubMed] [Google Scholar]
- 65.Rigby M. C., Hechinger R. F., Stevens L. 2002. Why should parasite resistance be costly? Trends Parasitol. 18, 116–120 10.1016/S1471-4922(01)02203-6 (doi:10.1016/S1471-4922(01)02203-6) [DOI] [PubMed] [Google Scholar]
- 66.Moret Y., Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168 10.1126/science.290.5494.1166 (doi:10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 67.Weis A. E., Hochberg M. E. 2000. The diverse effects of intraspecific competition on the selective advantage to resistance: a model and its predictions. Am. Nat. 156, 276–292 10.1086/303386 (doi:10.1086/303386) [DOI] [PubMed] [Google Scholar]
- 68.Polley H. W., Detling J. K. 1988. Herbivory tolerance of Agropyron smithii populations with different grazing histories. Oecologia 77, 261–267 10.1007/BF00379196 (doi:10.1007/BF00379196) [DOI] [PubMed] [Google Scholar]
- 69.Dyer M. I., Acra M. A., Wang G. M., Coleman D. C., Freckman D. W., McNaughton S. J., Strain B. R. 1991. Source–sink carbon relations in 2 Panicum coloratum ecotypes in response to herbivory. Ecology 72, 1472–1483 10.2307/1941120 (doi:10.2307/1941120) [DOI] [Google Scholar]
- 70.Roy B. A., Kirchner J. W., Christian C. E., Rose L. E. 2000. High disease incidence and apparent disease tolerance in a North American Great Basin plant community. Evol. Ecol. 14, 421–438 10.1023/A:1010997429365 (doi:10.1023/A:1010997429365) [DOI] [Google Scholar]
- 71.Castella G., Chapuisat M., Moret Y., Christe P. 2008. The presence of conifer resin decreases the use of the immune system in wood ants. Ecol. Entomol. 33, 408–412 10.1111/j.1365-2311.2007.00983.x (doi:10.1111/j.1365-2311.2007.00983.x) [DOI] [Google Scholar]
- 72.Simone M., Evans J. D., Spivak M. 2009. Resin collection and social immunity in honey bees. Evolution 63, 3016–3022 10.1111/j.1558-5646.2009.00772.x (doi:10.1111/j.1558-5646.2009.00772.x) [DOI] [PubMed] [Google Scholar]
- 73.Walbot V. 1985. On the life strategies of plants and animals. Trends Genet. 1, 165–169 10.1016/0168-9525(85)90071-X (doi:10.1016/0168-9525(85)90071-X) [DOI] [Google Scholar]
- 74.Birnbaum K. D., Sánchez Alvarado A. 2008. Slicing across kingdoms: regeneration in plants and animals. Cell 132, 697–710 10.1016/j.cell.2008.01.040 (doi:10.1016/j.cell.2008.01.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hart B. L. 1990. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294 10.1016/S0149-7634(05)80038-7 (doi:10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]