Abstract
Both host susceptibility and parasite infectivity commonly have a genetic basis, and can therefore be shaped by coevolution. However, these traits are often sensitive to environmental variation, resulting in genotype-by-environment interactions. We tested the influence of temperature on host–parasite genetic specificity in the Daphnia longispina hybrid complex, exposed to the protozoan parasite Caullerya mesnili. Infection rates were higher at low temperature. Furthermore, significant differences between host clones, but not between host taxa, and a host genotype-by-temperature interaction were observed.
Keywords: coevolution, Daphnia, genotype-by-environment interaction, host–parasite
1. Introduction
Host and parasite populations are predicted to maintain genetic variability through time-lagged negative frequency-dependent selection (Red Queen oscillations; [1]). However, evidence for coevolutionary dynamics from natural populations is limited, with few well-documented cases. One of the strongest examples of Red Queen operating in the wild involves a freshwater snail, Potamopyrgus antipodarum, and its trematode parasite. Within 10 years, common snail clones were replaced by formerly rare clones, and currently common ones became more susceptible to infection [2]. Furthermore, negative frequency-dependent selection was also found for the crustacean Daphnia magna and its bacterial parasite [3]. Otherwise, the observation of parasite-driven coevolutionary dynamics in nature is still rare (reviewed in [4]). In most long-term studies, the observed changes in host frequencies were either inconsistent (e.g. [5]) or opposite to the Red Queen predictions (e.g. [6,7]).
The scarcity of evidence for negative frequency-dependent selection in nature might be owing to environmental factors altering the genetic specificity of host–parasite interactions (see [4]), specificity being an important prerequisite for Red Queen oscillations [8]. If variable environmental conditions modify host–parasite genetic specificity, the net effect of frequency-dependent selection at the population level might differ from predictions under constant laboratory conditions [9]. Several studies have examined the influence of environment on the outcome of infection among multiple host or parasite genotypes but few have tested for three-way interactions (host-genotype × parasite-genotype × environment, reviewed in [10]). Temperature is a major environmental factor, and can affect the physiology, biochemistry and behaviour of hosts and parasites [9]. For example, a recent study on D. magna incorporated all possible interactions between host-genotype, parasite-genotype and temperature [11].
The specificity of host–parasite interactions was also shown to operate between groups of genotypes belonging to hybrid and parental taxa [12,13]. About 10 per cent of animal species are involved in hybridization and potential introgression [14]. Nonetheless, little is known about the influence of environment on the infection outcome in hybrid host–parasite systems. Consequently, it is important to understand how fluctuating environments influence specificity of host–parasite interactions at both the genotype and taxon level, as this might help to explain the uneven distribution of hybrids in so-called hybrid zones [15]. We studied this question using the Daphnia longispina hybrid complex, which dominates large European lakes, and its common and virulent protozoan parasite Caullerya mesnili [12].
This study aims to test whether temperature influences the infection outcome between six host genotypes, representing Daphnia hybrid and parental taxa, and two naturally co-occurring parasite isolates.
2. Material and methods
(a). System
Daphnia were isolated from Greifensee (Switzerland), in the summers of 2002–2004, except that clone 401 was isolated in winter, and B39 was hatched from a diapausing egg. Based on species-specific allozymes [12], three clones per Daphnia galeata and D. galeata × longispina taxon (nomenclature according to [16]) were selected (adequate for the detection of taxon differences; see [17]). Additionally, multi-locus-genotypes were determined using 15 unlinked microsatellite markers [18].
For primary infection, 50 heavily Caullerya-infected Daphnia were collected from Greifensee during 2006 summer epidemics and homogenized to obtain a spore-suspension. One D. galeata (G68) and one hybrid clone (344) were exposed to this mixture of Caullerya genotypes and kept separately for about 50 host generations (calculated with an estimated Daphnia generation time of 15 days under an average cultivating temperature of 16°C). Thus, we expected each of the parasite isolates to adapt to its host clone.
(b). Experiment
Prior to the experiment, the parasite laboratory cultures as well as the six experimental Daphnia clones were kept for five generations under standardized conditions. They were fed chemostat-grown Scenedesmus obliquus daily (1 mg C l−1), and kept in 0.45 µm filtered lake water at 16°C on a 16 L : 8 D regime. Experimental neonates (less than 24 h) from the second or third clutch were randomly distributed among treatments. Thus, six genetically distinct clones (three of each two taxa) were infected with two parasite isolates at two temperatures (12°C and 20°C) and replicated eight times, leading to 192 experimental units.
On day 1, each jar was filled with 4 ml water and a single Daphnia. On day 2, heavily infected Daphnia were collected from laboratory cultures and homogenized in distilled water. These spore-suspensions were distributed among all jars (0.2 heavily infected Daphnia each). Daphnia galeata clone G68 was used as a negative control, receiving no extra treatment.
From the third day of the experiment on, all animals were checked daily for survival and reproduction. Dead animals were examined for Caullerya spore-clusters. Experimental manipulations were carried out at degree-days, the product of day and temperature [19]. Every 80 degree-days, water was increased by 20 ml until it reached 100 ml per jar. Subsequently, water was fully changed every 80 degree-days, or after a new clutch. The experiment was terminated on degree-day 360 and all individuals were checked for Caullerya infection. The experimental units were classified as ‘infected’, or ‘non-infected’ based on the presence/absence of spores, when they died or when the experiment was terminated. Moreover, only animals that survived day 9 post infection, when Caullerya infections are first detectable, were included in the analyses, which were therefore carried out on 148 animals. No control became infected.
(c). Analyses
To analyse the infection outcome (i.e. presence/absence of spores), we fitted a generalized linear model (JMP 8.0) with bias reduction [20], binomial distribution and a logit link-function. Main effects included host taxon, clone (nested within taxon), temperature and parasite isolate. Host fecundity, the total number of offspring, was studied with a univariate general linear model and time to host death (in degree-days) using Cox-regression (SPSS). To visualize how clones grouped according to their microsatellite signature, a factorial correspondence analysis was performed [21].
3. Results
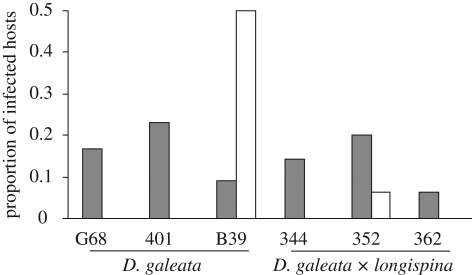
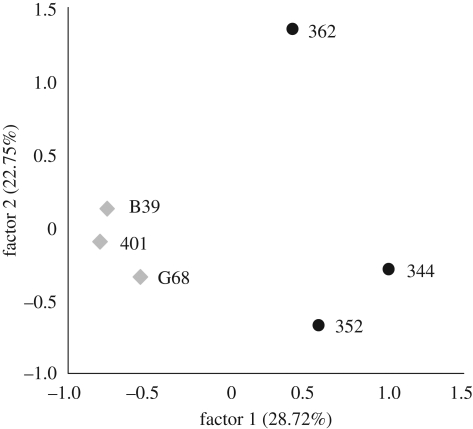
Both temperature and host clone (nested within taxon) significantly affected infection outcome. A higher proportion of Daphnia were infected at 12°C compared with the 20°C treatment (table 1 and figure 1). Further, there was a clone-by-temperature effect, with all clones but one showing higher infection levels at 12°C. The taxon and the parasite isolate effects and their interactions were not significant. Successful Caullerya infection resulted in host fecundity reduction by 97 per cent at 20°C and 77.4 per cent at 12°C. Moreover, host survival of Caullerya infected animals was more than 4.5 fold reduced compared with that of the uninfected individuals; both effects were significant (table 1). The genetic analyses showed a clustering pattern by taxon, with hybrids being genetically more diverse than parental clones (figure 2).
Table 1.
Results for the effect of temperature, Caullerya isolate, Daphnia taxon and clone, on the infection outcome and host life-history. (Non-significant interaction terms (numbers in italics, p > 0.05) were removed.)
| d.f. | F or χ2 | p-value | |
|---|---|---|---|
| infection outcome | |||
| temperature | 1 | 6.41 | 0.011 |
| parasite | 1 | 0.06 | 0.815 |
| taxon | 1 | 1.06 | 0.303 |
| clone (taxon) | 4 | 10.50 | 0.033 |
| temperature × clone (taxon) | 4 | 9.56 | 0.049 |
| host fecundity | |||
| temperature | 1 | 86.24 | <0.001 |
| parasite | 1 | 0.03 | 0.877 |
| clone | 5 | 7.79 | <0.001 |
| infection | 1 | 9.84 | 0.002 |
| temperature × clone | 5 | 12.10 | <0.001 |
| host survival | |||
| parasite | 1 | 3.04 | 0.083 |
| clone | 5 | 0.79 | 0.006 |
| infection | 1 | 33.04 | <0.001 |
Figure 1.
Susceptibility of Daphnia clones to Caullerya infection at 20°C (in white) and 12°C (in grey). For each clone, replicates infected with both Caullerya isolates were pooled. Only animals that survived day 9 post-infection, when infection is first visible, are included.
Figure 2.
The first two axes of the factorial correspondence analysis based on 15 microsatellite markers. Grey diamonds, D. galeata hosts; black circles, D. galeata × longispina.
4. Discussion
Temperature significantly affected Daphnia infection: a higher proportion of parasite-exposed Daphnia became infected at 12°C compared with the 20°C treatment. This strong effect of temperature is consistent with previous studies [22]. There was also genotype-by-environment effect: contrary to all other clones, clone B39 was more susceptible at 20°C. This raises the possibility that host genotypes will differentially suffer the effects of parasitism depending on how temperature fluctuates. Such effects can lead to the maintenance of genetic polymorphisms within and between populations [23]. Since clone B39 was the only clone hatched from a sediment core, one could argue that it never underwent natural selection and thus shows this different infection pattern. However, its fitness (measured as size of first clutch) is not different from other clones (ANOVA: 20°C − F1,67 = 0.48, p = 0.49; 12°C − F1,33 = 0.28, p = 0.60; C. N. Schoebel 2010, unpublished data). Moreover, clone B39 does not diverge from the range of other clones with respect to their genetic signature (figure 2). Furthermore, no difference between susceptibilities was found between hatched versus planktonic host for another Daphnia-microparasite system [24].
Interestingly, the direction of the temperature effect is opposite to all previous studies on D. magna and its bacterial parasite Pasteuria, where infection peaked at higher temperatures (e.g. [19]). In contrast to the D. longispina complex living in permanent lakes, D. magna inhabits temporary ponds of small size and rarely overwinters. Moreover, Pasteuria has its peak prevalence in summer [25], whereas Caullerya in winter [12]. Thus, Caullerya's higher infection success in the cold may reflect the parasite's thermal optimum. Alternatively, Daphnia immune functions might be less responsive at colder temperatures. In the Daphnia dentifera system inhabiting North American permanent lakes, the onset of epidemics caused by a yeast (Metschnikowia) also coincides with late-summer cooling of the lake [26]. This is owing to physical processes in a lake: decreasing temperature induces vertical water mixing, and consequently re-suspending parasite spores in the water column.
We did not detect a host taxon effect for the infection outcome, while there were differences among clones. Yet, microsatellite markers indicated greater genetic variation between, rather than within taxa. This contrast between variation at the phenotypic and at the genotypic level is consistent with another study on this hybrid complex [17].
We tested, but found no evidence for Caullerya adaptation to a particular host clone over approximately 50 host generations, even though rapid parasite adaptation was suggested by field data [12]. Conversely, parasite adaptation was found within five generations in the D. magna-Pasteuria system [27]. This difference is peculiar given that the Pasteuria study started with a single strain, whereas we used a diverse mixture of Caullerya spores, suggesting greater evolutionary potential. Nonetheless, this failure of the parasite to adapt to different host genotypes is consistent with findings for another Daphnia-parasite system [24].
In summary, the significant effect of temperature and the genotype-by-temperature interaction (with one clone being more susceptible at higher, whereas all others at lower temperature) demonstrate that the interaction of heterogeneous environments and infection has the potential to influence host population dynamics.
Acknowledgements
We thank Esther Keller for help throughout the experiment. Feedback from Tom Little, Otto Seppälä, Stuart Auld and anonymous reviewers improved this manuscript. Funding came from SNF, DFG and ETH-Zürich-CCES-Gedihap.
References
- 1.Bell G. 1982. The masterpiece of nature: the evolution and genetics of sexuality. Berkely, CA: University of California Press [Google Scholar]
- 2.Jokela J., Dybdahl M. F., Lively C. 2009. The maintenance of sex, clonal dynamics, and host–parasite coevolution in a mixed population of sexual and asexual snails. Am. Nat. 209, S43–S53 10.1086/599080 (doi:10.1086/599080) [DOI] [PubMed] [Google Scholar]
- 3.Decaestecker E., Gaba S., Raeymaekers J. A. M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L. 2007. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature 450, 870–873 10.1038/nature06291 (doi:10.1038/nature06291) [DOI] [PubMed] [Google Scholar]
- 4.Little T. J. 2002. The evolutionary significance of parasitism: do parasite-driven genetic dynamics occur ex silico? J. Evol. Biol. 15, 1–9 10.1046/j.1420-9101.2002.00366.x (doi:10.1046/j.1420-9101.2002.00366.x) [DOI] [Google Scholar]
- 5.Little T. J., Ebert D. 1999. Associations between parasitism and host genotype in natural populations of Daphnia (Crustacea: Cladocera). J. Anim. Ecol. 68, 134–149 [Google Scholar]
- 6.Henter H. J., Via S. 1995. The potential for coevolution in a host–parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution 49, 427–438 [DOI] [PubMed] [Google Scholar]
- 7.Vernon J. G., Okamura B., Jones C. S., Noble L. R. 1996. Temporal patterns of clonality and parasitism in a population of freshwater bryozoans. Proc. R. Soc. Lond. B 263, 1313–1318 10.1098/rspb.1996.0192 (doi:10.1098/rspb.1996.0192) [DOI] [PubMed] [Google Scholar]
- 8.Nee S. 1989. Antagonistic co-evolution and the evolution of genotypic randomization. J. Theoret. Biol. 140, 499–518 [DOI] [PubMed] [Google Scholar]
- 9.Thomas M. B., Blanford S. 2003. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 18, 344–350 10.1016/S0169-5347(03)00069-7 (doi:10.1016/S0169-5347(03)00069-7) [DOI] [Google Scholar]
- 10.Wolinska J., King K. C. 2009. Environment can alter selection in host–parasite interactions. Trends Parasitol. 25, 236–244 10.1016/j.pt.2009.02.004 (doi:10.1016/j.pt.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 11.Vale P. F., Little T. J. 2009. Measuring parasite fitness under genetic and thermal variation. Heredity 103, 102–109. (doi:10.1038/hdy.2009.54) [DOI] [PubMed] [Google Scholar]
- 12.Wolinska J., Bittner K., Ebert D., Spaak P. 2006. The coexistence of hybrid and parental Daphnia: the role of parasites. Proc. R. Soc. B 273, 1977–1983 10.1098/rspb.2006.3523 (doi:10.1098/rspb.2006.3523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolinska J., Lively C. M., Spaak P. 2008. Parasites in hybridizing communities: the Red Queen again? Trends Parasitol. 24, 121–126 10.1016/j.pt.2007.11.010 (doi:10.1016/j.pt.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 14.Mallet J. 2005. Hybridization as an invasion of the genome. Trends Ecol. Evol. 20, 229–237 10.1016/j.tree.2005.02.010 (doi:10.1016/j.tree.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 15.Moulia C. 1999. Parasitism of plant and animal hybrids: are facts and fates the same? Ecology 80, 392–406 10.1890/0012-9658(1999)080 (doi:10.1890/0012-9658(1999)080) [DOI] [Google Scholar]
- 16.Petrusek A., Hobæk A., Nilssen J. P., Skage M., Černý M., Brede N., Schwenk K. 2008. A taxonomic reappraisal of the European Daphnia longispina complex (Crustacea, Cladocera, Anomopoda). Zool. Scr. 37, 507–519 10.1002/jez.550 (doi:10.1002/jez.550) [DOI] [Google Scholar]
- 17.Rellstab C., Spaak P. 2009. Lake origin determines Daphnia population growth under winter conditions. J. Plankt. Res. 31, 261–271 10.1093/plankt/fbn120 (doi:10.1093/plankt/fbn120) [DOI] [Google Scholar]
- 18.Brede N., Thielsch A., Sandrock C., Spaak P., Keller B., Streit B., Schwenk K. 2006. Microsatellite markers for European Daphnia. Mol. Ecol. Notes 6, 536–539 10.1111/j.1471-8286.2005.01218.x (doi:10.1111/j.1471-8286.2005.01218.x) [DOI] [Google Scholar]
- 19.Mitchell S. E., Rogers E. S., Little T. J., Read A. F. 2005. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59, 70–80 10.1111/j.0014-3820.2005.tb00895.x (doi:10.1111/j.0014-3820.2005.tb00895.x) [DOI] [PubMed] [Google Scholar]
- 20.Firth D. 1993. Bias reduction of maximum likelihood estimates. Biometrika 80, 27 [Google Scholar]
- 21.Belkhir K., Borsa P., Chikhi L. N. 1996–2004. GENETIX. See http://www.genetix.univ-montp2.fr/genetix/genetix.htm. [Google Scholar]
- 22.Blanford S., Thomas M. B., Pugh C., Pell J. K. 2003. Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecol. Lett. 6, 2–5 10.1046/j.1461-0248.2003.00387.x (doi:10.1046/j.1461-0248.2003.00387.x) [DOI] [Google Scholar]
- 23.Laine L. 2008. Temperature-mediated patterns of local adaptation in a natural plant–pathogen metapopulation. Ecol. Lett. 11, 327–337 10.1111/j.1461-0248.2007.01146.x (doi:10.1111/j.1461-0248.2007.01146.x) [DOI] [PubMed] [Google Scholar]
- 24.Duffy M. A., Sivars-Becker L. 2007. Rapid evolution and ecological host-parasite dynamics. Ecol. Lett. 10, 44–53 10.1111/j.1461-0248.2006.00995.x (doi:10.1111/j.1461-0248.2006.00995.x) [DOI] [PubMed] [Google Scholar]
- 25.Duncan A. B., Little T. J. 2007. Parasite-driven genetic change in a natural population of Daphnia. Evolution 61, 796–803 10.1111/j.1558-5646.2007.00072.x (doi:10.1111/j.1558-5646.2007.00072.x) [DOI] [PubMed] [Google Scholar]
- 26.Caceres C. E., Hall S. R., Duffy M. A., Tessier A. J., Helmle C., MacIntyre S. 2006. Physical structure of lakes constrains epidemics in Daphnia populations. Ecology 87, 1438–1444 10.1890/0012-9658.x (doi:10.1890/0012-9658.x) [DOI] [PubMed] [Google Scholar]
- 27.Little T. J., Watt K., Ebert D. 2006. Parasite-host specificity: experimental studies on the basis of parasite adaptation. Evolution 60, 31–38 10.1111/j.0014-3820.2006.tb01079.x (doi:10.1111/j.0014-3820.2006.tb01079.x) [DOI] [PubMed] [Google Scholar]