Fig. 3.
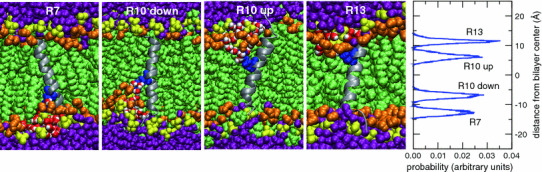
The four leftmost panels depict snapshots of equilibrated helical TM configurations of GGPGL6RL13GPGG (R7), GGPGL9RL10GPGG (R10), and GGPGL12RL7GPGG (R13) in a POPC bilayer. The peptide backbone is drawn as a gray ribbon, and the Arg side chains are colored blue. The lipids and water molecules are colored as in Fig. 1. Two initial conditions (“up” and “down”) were used for the R10 peptide, in which the Arg residue is in the middle of the sequence of the TM segment. The rightmost panel shows histograms of the position of the center of charge of the Arg centers of charge along the bilayer normal. In each peptide, the Arg residue snorkels toward the membrane–water interface to participate in interactions with lipid phosphate groups and water molecules (Color figure online)
