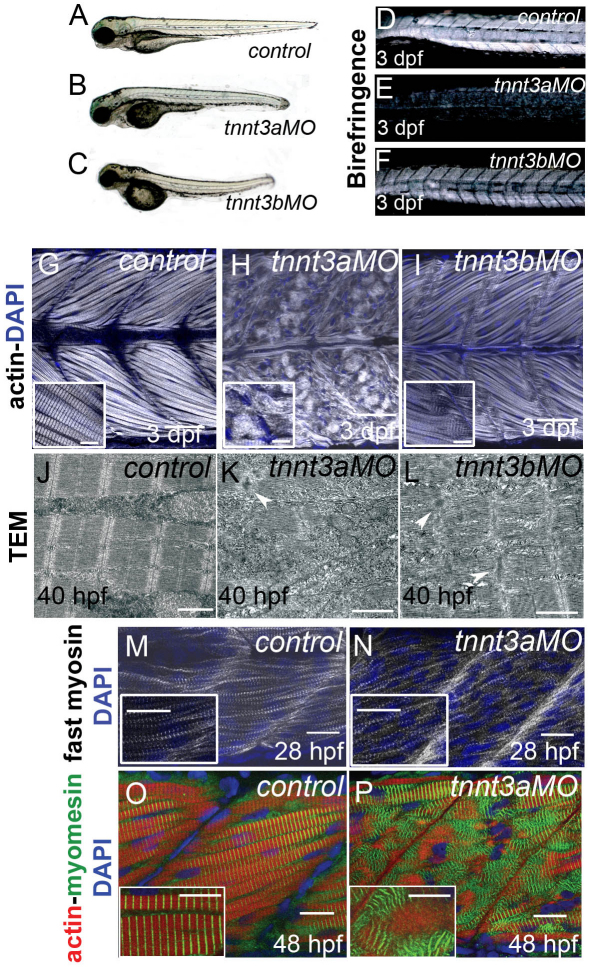
Fig. 4.
Myofibrils degrade in fast-twitch muscles of tnnt3-depleted embryos. (A–C) 3 dpf control (A), tnnt3a MO-injected (B) and tnnt3b MO-injected (C) embryos. (D–F) When trunks of the same embryos are observed under polarised light, embryos injected with tnnt3a MO (E) and tnnt3b MO (F) show total and partial loss of birefringence, respectively. (G–I) Lateral views of rostral somites in 3 dpf embryos. Confocal images from a plane deep in the trunk musculature show that actin is highly organised in fast muscle of control embryos (G), but appears completely disorganised in tnnt3a MO-injected embryos (H) and partially disorganised in tnnt3b MO-injected embryos (I; the inset shows a magnification of the myotendinous junction where actin localised in vertical Z lines or in horizontal stretched, non-striated filaments gives rise to a characteristic chequered appearance). Phalloidin is white and nuclei are counterstained with DAPI (blue). Scale bars: 50 μm; 10 μm in insets. (J–L) TEM images of fast-twitch muscles in 40 hpf control (J), tnnt3a MO- (K) and tnnt3b MO-injected embryos (L) reveal similar loss of sarcomeric integrity in the MO-injected embryos, with stacks of thick filaments and accumulations of Z line proteins (white arrowheads). Scale bars: 1 μm. (M,N) Forming fast-twitch fibres appear striated in both in control (M) and tnnt3a MO-injected (N) embryos at 28 hpf. Fast myosin is white and nuclei are blue. Scale bars: 20 μm. (O,P) At 48 hpf, M lines stained by myomesin (green) are regularly intercalated with thin filaments (phalloidin, red) in control embryos (O), whereas they cluster in groups and are excluded from actin-rich areas in tnnt3a MO-injected embryos (P, inset shows clusters). Scale bars: 15 μm; 10 μm in inset. M–P are lateral views of a single myotome, nuclei are stained with DAPI (blue).