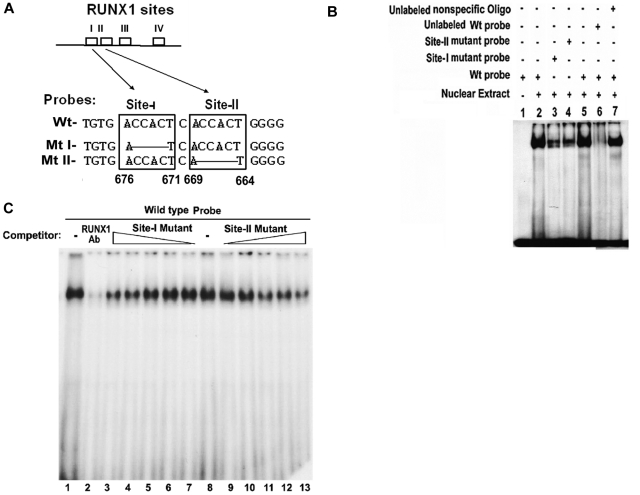
Figure 2.
EMSA using Wt oligonucleotide probe with sites I (−676/−671) and II (−669/−664) in MYL9 promoter and nuclear extract from PMA-treated HEL cells. (A) Wt −680/−660 bp) probe and Mt probes containing mutated RUNX1 sites I and II. (B) EMSA using Wt and Mt oligos (lanes 1-7). Lane 1, no extract; lanes 2 and 5, protein binding to Wt probe; lanes 3-4, reduced binding with Mt I and Mt II probes; lane 6, loss of binding by competition with unlabeled Wt probe; lane 7, no loss of binding by competition with nonspecific oligo. (C) Effect of anti-RUNX1 antibody (lanes 1 and 2) and of increasing amounts of unlabeled mutant probes I (lanes 7 to 3) and II (lanes 9 to 13) on protein binding to Wt oligo (−680/−660 bp). lane 1, protein binding to Wt probe; lane 2, loss of binding with anti-RUNX1 antibody. Lane 8 shows binding to Wt probe. Lanes 7 to 3, competition with increasing amounts of site I mutant probe; lanes 9-13, competition with increasing amounts of site II mutant probe. There is decreasing protein binding to Wt probe with increasing amount of either mutant I or mutant II probe, indicating that both sites are involved in protein binding to DNA.