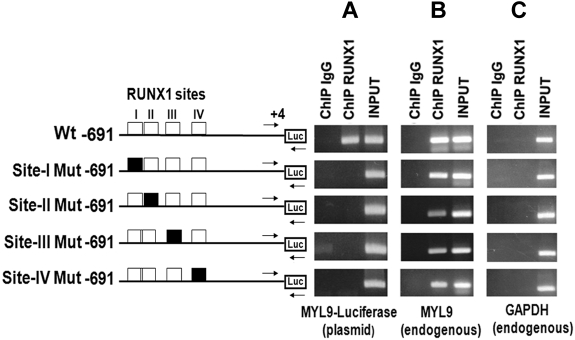
Figure 5.
Transient ChIP analysis using Wt and Mt MYL9 promoter (−691/+4)-luciferase reporter constructs in PMA-treated HEL cells. Cells were transfected with reporter constructs containing WtMYL9 promoter region (−691/+4) with RUNX1 sites (open boxes) or with RUNX1 sites I-IV individually mutated (filled boxes) as shown. ChIP was performed on these cells using anti-RUNX1 antibody or control IgG. PCR was performed using primers (shown by arrows), where the forward primer was specific to the MYL9 region (−140/−107) and the reverse primer was specific to the luciferase gene (Panel A; MYL9-luciferase plasmid). As a control, PCR was performed using a second set of primers (not shown) where both primers were specific to the endogenous MYL9 sequence (Panel B; endogenous MYL9). In addition, GAPDH was amplified (C). Each panel shows PCR products obtained from amplification of IgG- or anti-RUNX1 antibody-immunoprecipitated samples, and of input DNA. (A) Enrichment (RUNX1 antibody) of the MYL9-luciferase plasmid is observed from the cells transfected with Wt plasmid containing intact RUNX1 sites. This was abolished with mutations in any of the 4 RUNX1 sites. (B) As expected, Wt endogenous MYL9 sequence was amplified from chromatin immunoprecipitated by anti-RUNX1 antibody from all transfections, Wt or Mt. (C) GAPDH was not amplified from immunoprecipitated chromatin from any of the transfections.