Abstract
Background
Percutaneous coronary intervention (PCI) has witnessed rapid technological advancements resulting in improved safety and effectiveness over time. Little, however, is known about the temporal impact on patient-reported symptoms and quality of life following PCI.
Methods and Results
Temporal trends in post-PCI symptoms were analyzed using 8879 consecutive patients enrolled in the National Heart, Lung and Blood Institute-sponsored Dynamic Registry (wave 1: 1997(bare metal stents), wave 2: 1999 (uniform use of stents), wave 3: 2001 (brachytherapy), wave 4, 5: 2004, 2006 (drug eluting stents)). Patients undergoing PCI in the recent waves were older and more often reported comorbidities. However, fewer patients across the waves reported post-PCI angina at one year (wave 1–5: 24%, 23%, 18%, 20%, 20%; Ptrend:<0.001). The lower risk of angina in recent waves, however, was explained by patient characteristics including use of anti-anginal medications at discharge [relative risk (95% CI) for waves 2, 3, 4 vs 1: 1.0 (0.9–1.2), 0.9 (0.7–1.1), 1.0 (0.8–1.3), 0.9 (0.7–1.1)]. Similar trend was seen in the average quality of life scores over time (adjusted mean score for waves 1–5: 6.2, 6.5, 6.6 and 6.6; Ptrend: 0.01). Other factors associated with angina at one year included younger age, female gender, prior revascularization, need for repeat PCI and hospitalization for MI over one year.
Conclusion
Favorable temporal trends are seen in patient-reported symptoms following PCI in routine clinical practice. Specific subgroups, however, remain at risk for symptoms at one year and warrant closer attention.
Keywords: Percutaneous coronary intervention, temporal trend, angina, registries
BACKGROUND
Percutaneous coronary intervention (PCI) has now been in use for three decades, evolving rapidly with new devices and adjunct therapy. The field has also witnessed widening of profile of patients (and lesions) undergoing the procedure but with a concomitant rise in success rates and reduced need for repeat revascularization (1), (2). Although the two main goals of PCI are prolongation of life and/or improvement in health status, much focus to date has been placed on the former outcome. Indicators of health status have been shown to carry equal or greater importance than traditional risk factors for mortality in CVD (3). Though favorable impact of PCI on these ‘soft’ endpoints have been previously documented (4), (5), (6), information on temporal trends is lacking. Moreover, durability of symptom-relief with PCI is often attributed to subsequent repeat interventions and reliance on anti-anginal medications (7), (8). However, given the documented reduction in repeat interventions in more recent times, trends in the need and type of supplemental therapy need evaluation. The National Heart, Lung and Blood Institute (NHLBI)-sponsored multicenter Dynamic Registry comprises sequential waves of patients undergoing PCI in North America that span from the era of the bare metal stents (BMS) to that of the drug-eluting stents (DES). As such, these waves mark important advancements in the field and are ideal to evaluate temporal trends in, and predictors of, post-PCI health status in real-world practice.
METHODS
The prospective, multicenter NHLBI-sponsored Dynamic Registry enrolled consecutive patients undergoing PCI in clinical centers in North America (1), (2). Consecutive enrollment at each center ended once 200 white men and women were enrolled at that site or 1,600 white patients were enrolled across all sites. Then, consecutive minority patients were enrolled until approximately 2,000 patients had been enrolled across all sites in the pre-specified time intervals or ‘waves’ (consecutive enrollment dates: wave 1: July-November 1997, wave 2: February-April 1999, wave 3: October-December 2001, wave 4: February-April 2004, wave 5: February-May 2006). The research coordinators responsible for data collection participated in a training session prior to the start of each recruitment wave where the standardized forms and manual of operations are reviewed to ensure consistency in data collection practices. All clinical data collection was performed by the center coordinators via chart review for procedural data and telephone interview, mail questionnaires or during clinic visits for follow-up data using standardized report forms, guided by a manual of operations and definitions. Written informed consent was obtained for contact after discharge. Information on angina and other health status measures were obtained using brief questions based on reliable questionnaires well-suited for telephone-based interviews (9), (10). At the time of follow up, patients were asked if they had chest pain or discomfort (anginal symptoms) in the past six weeks, and when present, to describe the severity and frequency of the symptoms. Follow-up angina was then classified by the coordinators as stable or unstable by use of the definitions below. Patients were also asked to self-categorize their level of activity at follow-up as sedentary, mild, moderate, or strenuous, and to self-rate both quality of life and their satisfaction with present quality of life on an 11-point scale (higher scores denoted better quality). Data on events (death, hospitalizations for angina, myocardial infarction (MI), bypass surgery (CABG), and repeat PCI) over one year were also ascertained during contact and medical records were reviewed whenever possible for patients requiring repeat hospitalization. The study protocol was approved by the Institutional review boards of the coordinating center (University of Pittsburgh) and all the clinical sites involved.
Definitions
Stable angina was defined as pain precipitated by exertion and relieved by rest and/or sublingual nitroglycerin, with no change in pattern or severity within 6 weeks before intervention (baseline) or in the previous 6 weeks (follow-up). Unstable angina was defined as either pain presenting at rest, or exertional pain of at least Canadian Cardiovascular Society (CCS) Class III that began or increased in severity at least one CCS Class in the 2 months before intervention or follow-up. Acute MI, in the first 2 waves, was defined by the documented presence of ≥2 of the following criteria: clinical symptoms, enzyme-level elevations, new wall-motion abnormalities, and ≥2 serial electrocardiogram tracings showing changes from baseline or serially in ST-T and/or new Q waves in ≥2 contiguous leads; for waves 3 and 4, it was based on either biochemical evidence of necrosis or serial ECG changes. Attempted lesions were considered complex if ≥ 1 of the following features were present: evidence of thrombus, calcified, at bifurcation, chronic total occlusions (as seen in PCI for reasons other than MI), and ostial lesions (11). Procedural complications considered included embolization, slow flow, side branch occlusion, abrupt closures and dissections. Angiographic success was classified as either partial (some but not all attempted lesions successfully treated) or total (all attempted lesions successfully treated). Procedural success was defined as achievement of either partial or total angiographic success without death, MI, or emergency CABG. Supplemental therapy following index PCI comprised of the following mutually exclusive groups: 1) Bypass surgery alone or with PCI, 2) Repeat PCI or, 3) pharmacological maintenance therapy only (PMT, ≥1 of beta-blockers, calcium channel blockers and long-acting nitrates).
Statistical Methods
Temporal trends in baseline patient characteristics and outcomes at one year were assessed using the Cochran-Armitage test for dichotomous variables (12) and the Jonckeheere-Terpstra test for continuous and nominal/ordinal variables (13). Differences in cumulative events rates (death, MI, death/MI, non-staged repeat PCI, bypass surgery) over one year across the five waves were evaluated using the log-rank test. Difference in baseline characteristics between those with and without angina information was assessed using the Wilcoxon test or Chi-square test as appropriate. Covariates for multivariable models were identified from factors that differed significantly between the waves and also associated with one year outcome using stepwise logistic regression models (Pentry ≤ 0.20, Pstay ≤ 0.10). Logistic regression models were also used to generate propensity scores from baseline and clinical characteristics to account for potential bias introduced by missing outcome data. Given the prospective nature of the analyses using data from multiple clinical sites, the relative risk (95%CI) of post-PCI angina in waves 2–5 (reference: wave 1) were estimated using generalized linear models specifying the binomial distribution and log link function (PROC GENMOD in SAS), so as to account for intra-site correlation while providing propensity score-weighted risk estimates. All analyses wereperformed with SAS version 9.1 (SAS Institute Inc, NC).
RESULTS
Compared to 1997–98 (wave 1), consecutive patients (N=8879) undergoing PCI in the more recent waves were older and more often reported comorbidities (hypertension, diabetes and severe non-cardiac conditions) and prior revascularization (PCI or CABG) (Table 1). The index PCI, in these patients, more often involved single vessels/lesions with a concurrent increase in overall stent use (67% in wave 1 to 96% in wave 4; 75% DES in wave 4 to 90% in wave 5). Procedural success was achieved more often with fewer complications in the more recent waves (Table 2). While discharge use of recommended medications (aspirin, antiplatelets, beta-blockers and lipid-lowering medications) increased over time, fewer patients were discharged on calcium channel blockers (% in waves 1–5: 36, 27, 21, 16, 16) and long acting nitrates (% in waves 1–5: 34, 28, 25, 16, 13); Ptrend: <0.001 for both. Univariate logistic regression models also showed that discharge use of medications were associated with follow-up angina as follows – aspirin (odds ratio [95%CI]: 0.8 [0.6–1.0]), beta-blockers (0.9 [0.8–1.0]), lipid lowering drugs (0.9 [0.8–1.0]), antiplatelets (0.7 [0.6–0.8]), ACE-I inhibitors (1.0 [0.9–1.2]), long-acting nitrates (1.8 [1.6–2.0]) and calcium-channel blockers (RR [95%CI]: 1.4 [1.3–1.6].
Table 1.
Temporal trends in baseline patient and procedural characteristics of consecutive PCI patients undergoing PCI in the NHBLI-sponsored Dynamic Registry
| Wave 1 (1997) | Wave 2 (1999) | Wave 3 (2001) | Wave 4 (2004) | Wave 5 (2006) | P trend* | |
|---|---|---|---|---|---|---|
| N=1932 | N=1564 | N=1657 | N=1852 | N=1874 | ||
|
Patient Characteristics | ||||||
| Age ≥65 years,% | 43 | 46 | 49 | 46 | 47 | 0.01 |
| Female,% | 31 | 30 | 33 | 28 | 31 | 0.14 |
| White,% | 87 | 90 | 89 | 84 | 85 | 0.003 |
| Prior MI,% | 39 | 32 | 27 | 27 | 22 | <0.001 |
| Prior CABG,% | 16 | 18 | 19 | 21 | 19 | 0.001 |
| Prior PCI,% | 29 | 30 | 33 | 32 | 34 | 0.04 |
| Severe non-cardiac disease,% | 29 | 34 | 38 | 37 | 34 | <0.001 |
| History of Diabetes, % | 26 | 26 | 28 | 33 | 34 | <0.001 |
| History of Heart failure,% | 9 | 9 | 12 | 9 | 10 | 0.9 |
| History of Hypertension, % | 59 | 63 | 73 | 77 | 77 | <0.001 |
| Ejection fraction, mean † | 55 | 54 | 52 | 52 | 53 | <0.001 |
| Baseline vessel disease, % | <0.001 | |||||
| Single | 45 | 47 | 40 | 36 | 37 | <0.001 |
| Double | 32 | 30 | 33 | 32 | 31 | |
| Triple | 21 | 23 | 28 | 33 | 32 | |
| Significant lesions, mean | 2.7 | 2.7 | 2.9 | 3.1 | 3 | <0.001 |
|
Procedural Characteristics, % | ||||||
| Primary reason for index PCI | <0.001 | |||||
| Stable Angina | 26 | 21 | 20 | 23 | 20 | |
| UA / AMI | 65 | 71 | 69 | 63 | 63 | |
| ACAD /Others | 9 | 8 | 10 | 12 | 16 | |
| Circumstances of index PCI | <0.001 | |||||
| Elective | 67 | 57 | 52 | 58 | 57 | |
| Urgent | 23 | 33 | 38 | 31 | 31 | |
| Emergent | 10 | 10 | 10 | 11 | 12 | |
| # of lesions attempted | ||||||
| One | 67 | 69 | 69 | 73 | 72 | <0.001 |
| ≥ 2 or more | 33 | 31 | 31 | 27 | 28 | |
| # of vessels attempted | ||||||
| 1 native vessel only | 83 | 83 | 82 | 80 | 79 | |
| 2 –3 native vessels only | 10 | 10 | 10 | 12 | 15 | |
| Graft +/− native vessels (s) | 7 | 7 | 8 | 8 | 6 | |
| Evidence of thrombus | 25 | 24 | 18 | 18 | 18 | <0.001 |
| Calcified lesions | 31 | 28 | 24 | 27 | 35 | 0.02 |
| Bifurcation lesions | 13 | 16 | 17 | 12 | 11 | 0.001 |
| Tortuous lesions | 19 | 32 | 25 | 27 | 27 | <0.001 |
| Type C lesions | 21 | 18 | 23 | 24 | 34 | <0.001 |
| Gp IIb/IIIa inhibitors during PCI | 23 | 32 | 52 | 35 | 38 | <0.001 |
| Antiplatelets during PCI | 51 | 42 | 66 | 86 | 84 | <0.001 |
| Overall stent use | 67 | 80 | 87 | 93 | 96 | <0.001 |
| Drug-eluting stents | n/a | n/a | n/a | 74 | 90 | |
CABG: coronary artery bypass grafting, MI: Myocardial infarction, PCI: Percutaneous coronary intervention;
P trend calculated using Cochran-Armitage test for dichotomous variables and Jonckeheere-Terpstra test for continuous & ordinal variables;
33% of data missing
Table 2.
Temporal trends in in-hospital outcomes and discharge characteristics of consecutive PCI patients undergoing PCI in the NHLBI-sponsored Dynamic Registry
| Characteristics, % | Wave 1 (1997) | Wave 2 (1999) | Wave 3 (2001) | Wave 4 (2004) | Wave 5 (2006) | Ptrend* |
|---|---|---|---|---|---|---|
| N=1793 | N=1417 | N=1379 | N=1657 | N=1657 | ||
| Angiographic success | 93 | 93 | 95 | 95 | 96 | <0.001 |
| Procedural success | 96 | 96 | 97 | 97 | 98 | 0.01 |
| Procedural complications† | 19 | 10 | 7 | 8 | 6 | <0.001 |
| In-hospital mortality | 0.5 | 0.6 | 0.5 | 0.5 | 0.2 | 0.20 |
| In-hospital MI | 3 | 3 | 2 | 3 | 2 | 0.12 |
| In-hospital CABG | 1.1 | 1.2 | 0.5 | 1.1 | 0.3 | 0.01 |
| Major entry site complication | 3 | 4 | 3 | 5 | 6 | <0.001 |
| Among those discharged alive | ||||||
| Mean length of stay, days | 2.5 | 2.2 | 2.1 | 2.0 | 1.9 | <0.001 |
| Discharge medication use, % | ||||||
| Aspirin | 94 | 93 | 95 | 97 | 98 | <0.001 |
| Beta-blockers | 65 | 70 | 77 | 82 | 83 | <0.001 |
| Long-acting nitrates | 34 | 28 | 25 | 16 | 13 | <0.001 |
| ACE-Inhibitors | 30 | 35 | 47 | 55 | 50 | <0.001 |
| Calcium channel blockers | 36 | 27 | 21 | 16 | 16 | <0.001 |
| Lipid lowering medications | 45 | 59 | 75 | 87 | 88 | <0.001 |
| Ticlopidine /Clopidogrel | 67 | 80 | 93 | 96 | 97 | <0.001 |
CABG: coronary artery bypass grafting, MI: Myocardial infarction;
P trend calculated using Cochran-Armitage test for dichotomous variables and Jonckeheere-Terpstra test for continuous & ordinal variables;
Procedural complications is defined as presence of slow flow, side branch occlusion, dissections, abrupt closures or embolization following index PCI
Temporal trends in post-PCI angina and other patient-reported outcomes at one year
Of the 8879 consecutive PCI patients who consented to follow-up at baseline, 337 died over one year (% in waves 1–5: 3.9, 4.5, 3.6, 4.2, 3.6; Plogrank: 0.64) and 579 were alive but missing angina information (% in waves 1–5: 3.6, 5.3, 13.9, 6.7, 5.1; Ptrend: 0.02). Significant differences were observed between those with and without angina information at one year. These included more patients with age < 65 years (66% vs 54%, P <0.001), history of diabetes (33 % vs. 29%, P: 0.04) or congestive heart failure (11% vs. 9%, P: 0.03) and fewer patients with complex lesions (51% vs. 58%, P: 0.003) among those with missing data. No significant differences, however, were seen in baseline disease burden (61% multivessel disease in both, P: 0.85), procedural success (96% vs. 97%, P: 0.09) or one year cumulative events rates of MI (4% vs. 5%, Plogrank: 0.48), repeat PCI (13% vs. 12%, Plogrank: 0.68) or CABG (3% vs. 4%, Plogrank: 0.22).
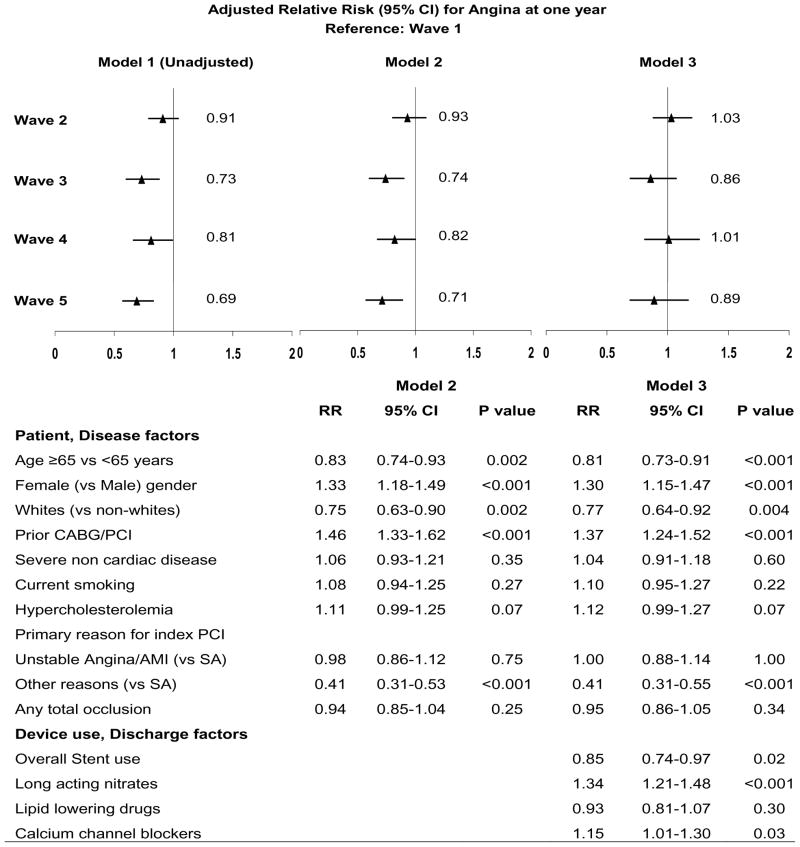
Of the remaining 7963 consecutive patients with information at one year, fewer patients over time reported anginal symptoms, both in the overall cohort (24% in wave 1 to 18% in wave 5, Ptrend: <0.001) as well as within specific indication subsets (Table 3). However, when present, the symptoms were reported to be more frequent in the recent waves (Ptrend: 0.03). QOL scores were significantly lower in symptomatic patients when compared to their asymptomatic counterparts in each wave (mean scores in waves 1–5 – Angina: 5.8, 5.8, 5.9, 6.2, 6.2 Ptrend: 0.004; No Angina: 7.1, 7.4, 7.4, 7.4, 7.2 Ptrend: 0.004). On average, QOL scores in the overall cohort were higher in the more recent waves, even after adjustment for age, sex, race and concomitant comorbidities (history of diabetes, hypercholesterolemia, hypertension, congestive heart failure, severe non-cardiovascular diseases). Figure 1 (Top panel) shows the propensity score-weighted relative risk estimates (unadjusted and adjusted) of angina at one year in the more recent waves when compared to wave 1. Compared to wave 1, the risk of angina is significantly lower in waves 3, 4 and 5, with no significant difference seen in wave 2 (Figure 1 model 1). While adjustment for baseline patient characteristics did not alter the pattern (Figure 1 model 2), further adjustment for the discharge use of long-acting nitrates and calcium –channel blockers attenuated the difference between the waves (Figure model 3). Pre-procedural factors that were significantly associated with angina at one year in the overall cohort include age (age < 65 years more than ≥65 years), gender (women more than men) and history of prior CABG or PCI (Figure 1 Bottom panel). While the overall use of stents was associated with a 15% lower risk of post-PCI angina, prescription of long-acting nitrates or calcium channel blockers at discharge was associated with higher odds of symptoms at one year. Although adjustment for intercurrent events over one year did not alter the pattern seen across the waves (data not shown), the need for repeat PCI (RR [95%CI]: 1.4 [1.2–1.6], P: <0.001) or MI over one year were associated with increased risk of angina (1.4 [1.2–1.7], P: <0.001) at one year; bypass surgery following index PCI, on the other hand, was associated with lower risk of angina (0.8 [0.7–1.0], P:0.05)
Table 3.
Temporal trends in angina and related characteristics at one year in consecutive PCI patients in the NHLBI-sponsored Dynamic Registry 1997–2004 cohorts
| Characteristics | Wave 1 (1997) | Wave 2 (1999) | Wave 3 (2001) | Wave 4 (2004) | Wave 5 (2006) | Ptrend* |
|---|---|---|---|---|---|---|
| N=1793 | N=1417 | N=1379 | N=1657 | N=1657 | ||
| Angina in past 6 weeks, % | ||||||
| Overall cohort | 24 | 23 | 18 | 20 | 18 | <0.001 |
| By 1° reason for initial PCI | ||||||
| Stable Angina | 27 | 26 | 20 | 24 | 22 | 0.03 |
| Unstable angina /AMI | 25 | 23 | 19 | 21 | 19 | <0.001 |
| Asymptomatic CAD / Other | 13 | 7 | 8 | 8 | 8 | 0.27 |
| Mean (adjusted) QOL scores † | 6.2 | 6.5 | 6.6 | 6.6 | 6.6 | <0.001 |
| Poor / Fair health status, % | 28 | 32 | 29 | 30 | 31 | |
| Physical Activity, % | 0.003 | |||||
| Sedentary | 9 | 10 | 10 | 10 | 11 | |
| Mild | 40 | 39 | 42 | 40 | 45 | |
| Moderate | 43 | 45 | 41 | 42 | 38 | |
| Strenuous | 7 | 7 | 7 | 9 | 6 | |
| Hospitalizations over 1 year, % | ||||||
| Death | 4 | 5 | 4 | 4 | 4 | 0.64 |
| Death/MI | 9 | 10 | 8 | 9 | 8 | 0.20 |
| Repeat PCI | 16 | 13 | 12 | 10 | 10 | <0.001 |
| CABG | 7 | 5 | 4 | 3 | 3 | <0.001 |
| One year medication use among those prescribed at discharge, % | ||||||
| Aspirin | 81 | 80 | 74 | 82 | 87 | <0.001 |
| Lipid lowering medications | 76 | 78 | 73 | 79 | 78 | 0.04 |
| Antiplatelets ‡ | 2 | 4 | 15 | 55 | 73 | <0.001 |
| ACE-Inhibitors | 59 | 61 | 59 | 64 | 64 | 0.02 |
| Beta blockers | 75 | 75 | 70 | 76 | 78 | 0.03 |
| Long-acting nitrates | 51 | 40 | 37 | 43 | 47 | 0.08 |
| Calcium channel blockers | 58 | 59 | 51 | 52 | 60 | 0.50 |
| Among those with angina | ||||||
| N=436 | N=321 | N=246 | N=332 | N=332 | ||
| Severity of symptoms, % | 0.06 | |||||
| Stable CHC I/II Angina | 55 | 54 | 60 | 59 | 49 | |
| Stable CHC III/IV Angina | 21 | 21 | 23 | 13 | 12 | |
| Unstable Angina/AMI | 25 | 25 | 17 | 29 | 40 | |
| Frequency of symptoms,% | 0.03 | |||||
| Three or more times/day | 4 | 8 | 6 | 10 | 11 | |
| One to two times/day | 13 | 10 | 14 | 13 | 11 | |
| Several times/week | 31 | 31 | 39 | 31 | 30 | |
| Once/week or less | 52 | 51 | 41 | 46 | 48 | |
| Sublingual NTG, % | 76 | 68 | 64 | 61 | 59 | <0.001 |
ACE: Angiotensin-converting enzyme inhibitors, ARB: Angiotensin-receptor blockers, CABG: coronary artery bypass grafting, MI: Myocardial infarction, NTG: Nitroglycerin, PCI: Percutaneous coronary intervention;
Ptrend calculated using Cochran-Armitage test for dichotomous, Jonckeheere-Terpstra test for ordinal variables and Log rank test for cumulative hospitalization rates over one year:
Test for trend done using linear contrasts (PROC GLM) adjusting for age, sex, race and concomitant comorbidities (history of diabetes, hypercholesterolemia, hypertension, congestive heart failure, severe non-cardiovascular diseases);
Information on 1-year Clopidogrel use not routinely collected in Waves 2 and 3
Figure 1.
Relative risk (95% confidence intervals) for angina at one year after PCI in the NHLBI-sponsored 1997–2006 Dynamic Registry waves
MI: Myocardial infarction; PCI: Percutaneous coronary intervention; PVD: Peripheral vascular disease, SA: Stable angina; UA; Unstable angina.
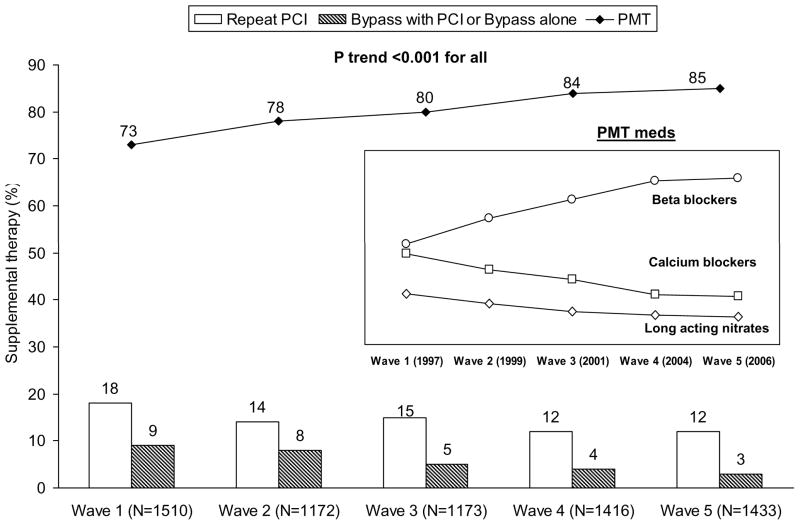
The percentage of patients reporting medication use at one year, when initially discharge on them, increased over time - aspirin (waves 1 to 5: 81% to 87%), beta-blockers (75% to 78%), ACE inhibitors (59% to 64%) and lipid lowering medications (76% to 78%). Figure 2 shows the temporal trends in the need for post-PCI supplemental therapy, arranged in the following order of aggressiveness (CABG alone or with repeat PCI > repeat PCI > only pharmacological maintenance therapy (PMT) of one or more of beta blockers or calcium channel blockers or nitrates). In the 1997–98 wave, approximately three out of four patients reported PMT use with no additional revascularization over a year. The proportion of PMT use increased with concomitant reductions in the need for repeat interventions (repeat PCI or CABG) across the waves. Specifically, within the PMT group, use of beta blockers increased while that of long-acting nitrates and calcium-channel blockers decreased over time (Figure 2 insert box). Interestingly, the adjusted mean QOL scores in the overall cohort differed by the type of supplemental therapy (PMT: 6.5, Repeat PCI: 6.0, CABG: 6.4; P: <0.001).
Figure 2.
Temporal trends in supplemental therapy* following index PCI in the NHLBI-sponsored 1997–2006 Dynamic Registry
* Supplemental therapy following index PCI comprised of the following mutually exclusive groups: 1) Bypass surgery alone or with PCI, 2) Repeat PCI or, 3) pharmacological maintenance therapy only (PMT, 1 of beta-blockers, calcium channel blockers and long-acting nitrates); †Ptrend calculated using the Jonckeheere-Terpstra test for ordinal variables; PMT: Pharmacological maintenance therapy ( 1 of beta-blockers, calcium channel blockers and long-acting nitrates)
DISCUSSION
Ever since its use in 1977 (14), PCI as a treatment modality has witnessed rapid technological advancements with a resultant widening in patient and lesion profiles. The effectiveness of the procedure in relieving symptoms and improving health status has been documented extensively but in specific time periods (5),(6), (7), (15). Temporal trends in routine practice of PCI are reflect higher success rates with significantly reduced need for repeat revascularization over time (2). The related impact on symptoms and quality of life, however, remains largely unknown. Our report provides a time-sensitive documentation of health status at one year after PCI in a prospective multicenter registry spanning a decade of clinical practice. Specifically, the risk of post-procedural angina, as perceived by patients, has decreased over time with fewer repeat interventions and reduced reliance on anti-anginal medications.
The NHLBI-sponsored Dynamic Registry was primarily initiated to provide a snapshot of PCI use in routine practice. As such, the Registry marks the uptake of key devices in the specific time periods – wave 1 (advent of BMS), wave 2 (uniform use of BMS), wave 3 (brachytherapy), wave 4 (advent of DES), and wave 5 (established use of DES) (1), (2). Development of these devices was principally aimed at reducing the need for repeat interventions, a goal that has indeed been met over time (2). The lack of apparent mortality benefit, on the other hand, is viewed in light of the sicker profile of treated patients. This being the case, the reported favorable impact on post-procedural angina speaks broadly to the progress in the field. Admittedly, the lack of baseline health status limits the ability to quantify any meaningful change as a result of the procedure. However, the reported trends are independent of the primary reason for index PCI, the closest representation of baseline symptom status in our cohort. These findings are also noteworthy given the increased use of selective revascularization strategies (more baseline disease but fewer attempts on ≥2 lesions) seen over time.
The ‘era effect’, as represented by the recruitment waves, can be considered a complex synergistic interaction of operator training, technology and secondary management. The observed trends coincide with improved procedural success, reduced need for long-acting nitrates and repeat revascularizations. Coronary revascularization offers a prime opportunity to ensure initiation of evidence-based secondary pharmacological therapy and as such, the observed temporal trends in use of these medications are encouraging. Adherence to risk factor management and specifically to discharge medications have been previously shown to influence symptoms (16) and long-term outcomes (17), (18). Therefore, the modest (and non-significant) effect of discharge use of individual medications (aspirin, beta-blockers, antiplatelets and lipid-lowering drugs) on symptoms at one year deserves mention. Interestingly, differences in cumulative event rates in the Lescol Intervention Prevention study, the landmark trial that established benefits of initiating post-PCI statin therapy, became more apparent after one year (19). Even in the more recent Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial, the use of optimal medical therapy resulted in narrowing of the significant difference in angina relief seen with PCI with longer follow-up (20). Thus, while it must be acknowledged that data on baseline use or risk factor control was not available in our Registry, it is possible that longer follow-up periods are required to appreciate distinct impact of pharmacotherapy on symptoms.
Durability of symptom-relief with PCI, either alone or when compared to CABG, has often been attributed to subsequent repeat interventions and reliance on anti-anginal medications (7), (8). The temporal variation in the use of supplemental therapy in our report, therefore, captures the overall impact of PCI evolution on subsequent patient symptoms. In the pre-stent era, approximately one in four patients, who underwent PCI outside of acute MI setting and were angina-free at one year, required repeat intervention (6% CABG and 19% repeat PCI) (21). Subsequent analysis of symptomatic patients in the NHLBI-sponsored Dynamic Registry showed lower repeat revascularization rates with an increase in use of maintenance therapy at one year (6). This trend has certainly improved over time with more patients reporting pharmacological therapy in the setting of fewer subsequent repeat interventions. Reliance on anti-anginal therapy following PCI has often been considered a limitation of the procedure and to this end, the drastic concomitant reduction in the use of long-acting nitrates and calcium channel blockers is encouraging.
The number of PCIs performed in the United States has increased by almost 300% since 1987 and therefore, reports of post-procedural symptoms, even if only in 18–25% of patients, demands enquiry. This becomes especially crucial given the absence of documented mortality benefit with PCI and symptom relief becomes the primary goal of the procedure. The impact of PCI on health status has been extensively studied (6), (22),(23),(24) and predictors of post-PCI symptoms identified (25). We extend these reports to present correlates of post-procedural symptoms after accounting for the evolution in the field. Patient factors associated with post-PCI angina, in models that adjusted for the ‘waves’, included female gender, younger age, severe concomitant non-cardiac comorbidities and history of prior revascularization. These findings are consistent with previous reports (6), (26), (27) and re-emphasize the need to explore potential mechanisms. For example, certain subgroups like women are prone to ischemia even in the absence of significant stenoses with endothelial dysfunction and other non-cardiac causes as plausible mechanisms (28). On the other hand, patients with prior and repeat revascularizations may be reflective of subsets with chronic disease burden not amenable/suitable for revascularization per se. Medical decision-making is a complex, multifactorial process with interplay of physician discretion, patient preference and treatment affordability. However, the above findings along with the small but significant increase in procedures performed for reasons other than angina or MI calls into question the appropriateness of PCI use, both from a clinical as well as economic standpoint.
Limitations
Patient-centered outcomes are gaining rapid attention both in the decision-making process as well as for assessment of procedural performance (29),(30). To this end, it is encouraging to see that the overall prevalence and risk of patient-perceived symptoms following PCI has decreased over time. The enrollment of consecutive patients with no exclusion criteria and the use of standardized data collection procedures across the waves strengthen these comparisons. Nevertheless, our results must be viewed in the context of the limitations inherent to use of a registry database and the potential effect of residual confounding cannot be ruled out. The NHLBI-sponsored Dynamic Registry was initiated to primarily provide a snapshot of PCI use as a treatment modality for coronary artery disease and as such, relied on voluntary participation of clinical centers. Majority of the sites, therefore, are medium to high volume hospitals and academic centers, thus limiting the generalizability of our findings. The Registry was also designed to allow for maximum data collection with efficient use of limited resources. Patient-reported outcomes in our registry, therefore, were captured only at follow up using brief questions leading to limited characterization. Even so, the association of symptoms with poor quality of life ratings supports the validity of our assessment in this cohort. Health status indicators are also influenced by social environment, relationships, personal values and emotions (31),(32), all details not collected in our registry. Future research, therefore, will focus on evaluating the long-term impact of the available measures, as a means to identifying their prognostic usefulness.
CONCLUSION
Our report documents favorable temporal trends in the prevalence and risk of post-PCI angina in a large prospective, multicenter registry of routine clinical practice. Post-procedural management in contemporary practice reflects greater use of recommended pharmacological therapy with fewer subsequent repeat interventions. Specific subgroups, however, continue to be associated with symptoms at one year and warrant closer attention.
What is known
In the last three decades, the field of percutaneous coronary intervention (PCI) has evolved rapidly with advancements in technology as well as adjunct and secondary pharmacological therapy.
Contemporary PCI practice includes more patients with severe comorbidities, acute coronary syndromes and multivessel disease, and yet, continues to achieve high procedural success rates with significant reductions in the need for repeat revascularization.
What this study adds
Temporal trends in patient-perceived symptoms following PCI are indicative of a reduction in the risk of post-procedural angina at one year of follow-up.
PCI patients today are managed more often with recommended pharmacological therapy (more beta-blocker use, less reliance on nitrates and calcium channel blockers), and less often require revascularization (repeat PCI or bypass surgery) after the index procedure.
Women, younger patients, those with prior revascularization or other non-cardiac comorbidities are more likely to report symptoms at one year, thus, warranting further investigation into the plausible underlying mechanisms in these subgroups.
Acknowledgments
Funding Source: This study was supported by grants (HL-33292-15 through HL-33292-22) from the NHLBI (Bethesda, Md).
Footnotes
Disclosures:
Lakshmi Venkitachalam: None
Kevin E Kip: None
Suresh R Mulukutla: None
Faith Selzer: None
Warren Laskey: None
James Slater: None
Howard A. Cohen: None
Robert L Wilensky: Grant support from Boston Scientific (<$10K) and Ownership Interests in Johnson & Johnson (>$10K)
David O. Williams: Grant support from Cordis Corporation, BostonScientific, and Abbott Vascular (all <$10K) and Consultant/advisory board of Cordis Corporation (<$10K);
Oscar C Marroquin: None
Kim Sutton-Tyrrell: None
Clareann H Bunker: None
Sheryl F Kelsey: None
References
- 1.Williams DO, Holubkov R, Yeh W, Bourassa MG, Al Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes DR, Jacobs A, Kelsey SF, King SB, III, Myler R, Slater J, Stanek V, Vlachos HA, Detre KM. Percutaneous coronary intervention in the current era compared with 1985–1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–2951. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 2.Venkitachalam L, Kip KE, Selzer F, Wilensky RL, Slater J, Mulukutla SR, Marroquin OC, Block PC, Williams DO, Kelsey SF for the Investigators of NHLBI-Sponsored. Twenty-Year Evolution of Percutaneous Coronary Intervention and Its Impact on Clinical Outcomes: A Report From the National Heart, Lung, and Blood Institute-Sponsored, Multicenter 1985–1986 PTCA and 1997–2006 Dynamic Registries. Circ Cardiovasc Intervent. 2009;2:6–13. doi: 10.1161/CIRCINTERVENTIONS.108.825323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health Status Predicts Long-Term Outcome in Outpatients With Coronary Disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 4.Parisi AF, Folland ED, Hartigan PA. A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease: Veterans Affairs ACME investigators. N Engl J Med. 1992;326 :10–16. doi: 10.1056/NEJM199201023260102. [DOI] [PubMed] [Google Scholar]
- 5.Rinfret S, Grines CL, Cosgrove RS, Ho KKL, Cox DA, Brodie BR, Morice MC, Stone GW, Cohen DJ. Quality of life after balloon angioplasty or stenting for acute myocardial infarction : One-year results from the Stent-PAMI trial. Journal of the American College of Cardiology. 2001;38:1614–1621. doi: 10.1016/s0735-1097(01)01599-6. [DOI] [PubMed] [Google Scholar]
- 6.Holubkov R, Laskey WK, Haviland A, Slater JC, Bourassa MG, Vlachos HA, Cohen HA, Williams DO, Kelsey SF, Detre KM. Angina 1 year after percutaneous coronary intervention: A report from the NHLBI Dynamic Registry. American Heart Journal. 2002;144:826–833. doi: 10.1067/mhj.2002.125505. [DOI] [PubMed] [Google Scholar]
- 7.Detre K, Holubkov R, Kelsey S, Bourassa M, Williams D, Holmes D, Jr, Dorros G, Faxon D, Myler R, Kent K. One-year follow-up results of the 1985–1986 National Heart, Lung, and Blood Institute’s Percutaneous Transluminal Coronary Angioplasty Registry. Circulation. 1989;80:421–428. doi: 10.1161/01.cir.80.3.421. [DOI] [PubMed] [Google Scholar]
- 8.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 9.ROSE GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 10.Visser MC, Koudstaal PJ, Erdman RA, Deckers JW, Passchier J, van Gijn J, Grobbee DE. Measuring quality of life in patients with myocardial infarction or stroke: a feasibility study of four questionnaires in The Netherlands. J Epidemiol Community Health. 1995;49:513–517. doi: 10.1136/jech.49.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilensky RL, Selzer F, Johnston J, Laskey WK, Klugherz BD, Block P, Cohen H, Detre K, Williams DO. Relation of percutaneous coronary intervention of complex lesions to clinical outcomes (from the NHLBI Dynamic Registry) Am J Cardiol. 2002;90:216–221. doi: 10.1016/s0002-9149(02)02457-8. [DOI] [PubMed] [Google Scholar]
- 12.Margolin BH. Test for Trend in Proportions. 1988:334–336. [Google Scholar]
- 13.Pirie W. Jonckheere Tests for Ordered Alternatives. 1983:315–318. [Google Scholar]
- 14.Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1:263. doi: 10.1016/s0140-6736(78)90500-7. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz HM, Cohen DJ, Williams C, Baim DS, Brinker J, Cabin HS, Heuser R, Hirshfeld J, Leon MB, Moses J, Savage MP, Cleman M. Health after coronary stenting or balloon angioplasty: results from the Stent Restenosis Study. Am Heart J. 1997;134:337–344. doi: 10.1016/s0002-8703(97)70065-6. [DOI] [PubMed] [Google Scholar]
- 16.Decker C, Ahmad H, Moreng KL, Maddox TM, Reid KJ, Jones PG, Spertus JA. Risk factor management after myocardial infarction: Reported adherence and outcomes. American Heart Journal. 2009;157:556–562. doi: 10.1016/j.ahj.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. American Heart Journal. 2008;155:772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Jaber WA, Lennon RJ, Mathew V, Holmes DR, Jr, Lerman A, Rihal CS. Application of Evidence-Based Medical Therapy Is Associated With Improved Outcomes After Percutaneous Coronary Intervention and Is a Valid Quality Indicator. Journal of the American College of Cardiology. 2005;46:1473–1478. doi: 10.1016/j.jacc.2005.06.070. [DOI] [PubMed] [Google Scholar]
- 19.Serruys PW, de Feyter PJ, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami M, Jackson G, Strauss B, Meier B, Lescol Intervention Prevention Study (LIPS) Investigators Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 20.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkowitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB COURAGE Trial Research Group. Effect of PCI on quality of life in patients with stable coronary artery disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 21.Detre KM, Takaro T, Hultgren H, Peduzzi P. Long-term mortality and morbidity results of the Veterans Administration randomized trial of coronary artery bypass surgery. Circulation. 1985;72:V84–V89. [PubMed] [Google Scholar]
- 22.Krumholz HM, McHorney CA, Clark L, Levesque M, Baim DS, Goldman L. Changes in health after elective percutaneous coronary revascularization. A comparison of generic and specific measures. Med Care. 1996;34:754–759. doi: 10.1097/00005650-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Borkon AM, Muehlebach GF, House J, Marso SP, Spertus JA. A comparison of the recovery of health status after percutaneous coronary intervention and coronary artery bypass. The Annals of Thoracic Surgery. 2002;74:1526–1530. doi: 10.1016/s0003-4975(02)04063-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Mahoney EM, Stables RH, Booth J, Nugara F, Spertus JA, Weintraub WS. Disease-Specific Health Status After Stent-Assisted Percutaneous Coronary Intervention and Coronary Artery Bypass Surgery: One-Year Results From the Stent or Surgery Trial. Circulation. 2003;108:1694–1700. doi: 10.1161/01.CIR.0000087600.83707.FD. [DOI] [PubMed] [Google Scholar]
- 25.Spertus JA, Salisbury AG, Jones PG, Conway DG, Thompson RC. Predictors of Quality-of-life Benefit after Percutaneous Coronary Intervention. Circulation. 2004;110:3789–3794. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 26.Kelsey SF, James M, Holubkov AL, Holubkov R, Cowley MJ, Detre KM. Results of percutaneous transluminal coronary angioplasty in women. 1985–1986 National Heart, Lung, and Blood Institute’s Coronary Angioplasty Registry. Circulation. 1993;87:720–727. doi: 10.1161/01.cir.87.3.720. [DOI] [PubMed] [Google Scholar]
- 27.Mullany CJ, Mock MB, Brooks MM, Kelsey SF, Keller NM, Sutton-Tyrrell K, Detre KM, Frye RL. Effect of age in the Bypass Angioplasty Revascularization Investigation (BARI) randomized trial. Ann Thorac Surg. 1999;67:396–403. doi: 10.1016/s0003-4975(98)01191-6. [DOI] [PubMed] [Google Scholar]
- 28.Shaw LJ, Lewis JF, Hlatky MA, Hsueh WA, Kelsey SF, Klein R, Manolio TA, Sharrett AR, Tracy RP Endorsed by the American College of Cardiology Foundation. Women’s Ischemic Syndrome Evaluation: Current Status and Future Research Directions: Report of the National Heart, Lung and Blood Institute Workshop: October 2–4, 2002: Section 5: Gender-Related Risk Factors for Ischemic Heart Disease. Circulation. 2004;109:56e–58. doi: 10.1161/01.CIR.0000116210.70548.2A. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Crossing the Quality Chasm: a New Health System for the Twenty-First Century. 2001 [Google Scholar]
- 30.Krumholz HM, Peterson ED, Ayanian JZ, Chin MH, DeBusk RF, Goldman L, Kiefe CI, Powe NR, Rumsfeld JS, Spertus JA, Weintraub WS. Report of the National Heart, Lung, and Blood Institute Working Group on Outcomes Research in Cardiovascular Disease. Circulation. 2005;111:3158–3166. doi: 10.1161/CIRCULATIONAHA.105.536102. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen SS, Ong ATL, Lemos PA, Erdman RAM, Serruys PW, van Domburg RT. Risk factors for impaired health status differ in women and men treated with percutaneous coronary intervention in the drug-eluting stent era. Journal of Psychosomatic Research. 2006;61:11–17. doi: 10.1016/j.jpsychores.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Denollet J, Pedersen SS, Ong ATL, Erdman RAM, Serruys PW, Van Domburg RT. Social inhibition modulates the effect of negative emotions on cardiac prognosis following percutaneous coronary intervention in the drug-eluting stent era. Eur Heart J. 2006;27:171–177. doi: 10.1093/eurheartj/ehi616. [DOI] [PubMed] [Google Scholar]