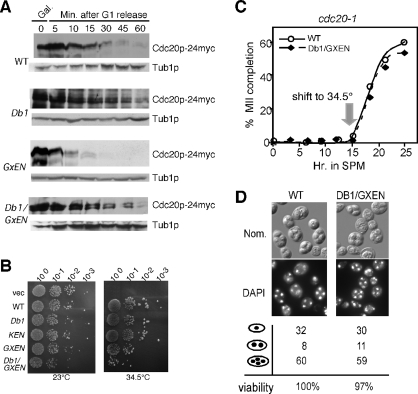
FIGURE 6:
The GxEN degron does not function in mitotic cells. (A) Wild-type cells (RSY10) harboring either WT (pUS995), Db1 (pKC5006), GxEN (pKC5009), or Db1/GxEN (pKC5016) galactose-inducible Cdc20p-24myc plasmids were grown in raffinose to mid-log phase, arrested in G1, and then induced by the addition 2% galactose. Degradation of Cdc20p was monitored by Western analysis at the time points indicated following G1 release. The blots were stripped and reprobed for Tub1p as loading control. (B) The Cdc20p Db1, KEN, GxEN, and Db1/GxEN mutants are functional. Mid-log phase cdc20-1 cultures (RSY809) containing either vector (pRS426) or galactose-inducible Cdc20p constructs (derived from functional Cdc20p-24myc CEN plasmid [pUS995] using the oligonucleotides described in Supplemental Table S2) were grown in raffinose, serial diluted (1:10), and spotted onto 2% galactose medium selecting for plasmid maintenance at 23°C (permissive) and the restrictive temperatures (34.5°C). Images were collected following 72 h incubation. (C) Meiotic progression was monitored in cdc20-1 cultures (RSY809) expressing either WT CDC20 (pKC5071) or the DB1/GxEN mutant (pKC5072) under the control of AMA1 promotor. The cells were induced to enter meiosis at the permissive temperature (23°C) and switched to the restrictive temperature after 15 h in SPM. The cells were analyzed by DAPI staining to monitor the appearance of tetranucleated cells. (D) Nomarski imaging (top) and DAPI staining (bottom) of cells described in (C) after 24 h in SPM. The percentage of mono-, bi-, and tetranucleated cells in each culture was determined by DAPI analysis. The relative spore viability was scored with the WT value set at 100%.