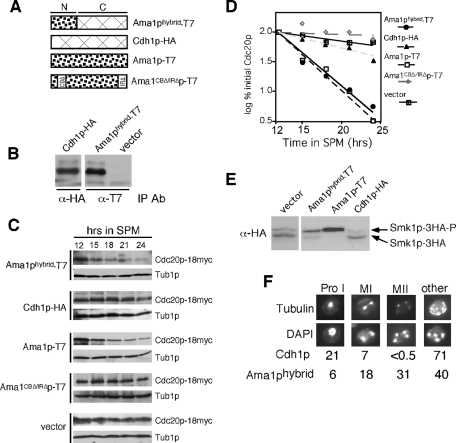
FIGURE 9:
The Ama1-Cdh1p fusion protein partially complements ama1Δ phenotypes. (A) Diagram depicting the proteins tested. N represents the amino-terminal divergent region, C the carboxyl terminus–conserved WD40 repeat domain. All constructs are under the control of the AMA1 promotor and terminator. (B) The expression of the AMA1-hybrid and AMA1pro-CDH1 constructs were verified in meiotic cultures. Extracts prepared from samples harvested 9 h following transfer to SPM were immunoprecipitated, and then Western blots were performed with the indicated antibodies. (C) Cdc20p levels were followed in meiotic RSY853 (ama1Δ, Cdc20-18myc) harboring either the Ama1-hybrid (pKC3077), Cdh1p-HA (pKC3078), Ama1p-T7 (pKC3056), or Ama1CboxΔ/IRΔp-T7 (pKC3057) expression plasmids or vector control. (D) The Cdc20p signals obtained in (C) were quantitated and graphed. All Cdc20p values were compared with Tub1p levels to control for protein quantitation. (E) Smk1p activation assays were performed on CMY15 containing either an empty vector, AMA1-T7, AMA1-hybrid, or AMA1pro-CDH1–expressing plasmids as described in Figure 3B. (F) An ama1Δ culture (RSY562) harboring plasmids expressing either Cdh1p-HA or Ama1p-hybrid was induced to enter meiosis and harvested for analysis after 12 h in SPM. Nuclei and spindle microtubules were visualized by DAPI (bottom) and indirect immunofluorescence (top), respectively. The number of cells seen at each stage of meiosis was scored as a percentage of the total cells counted. 1000× final magnification.