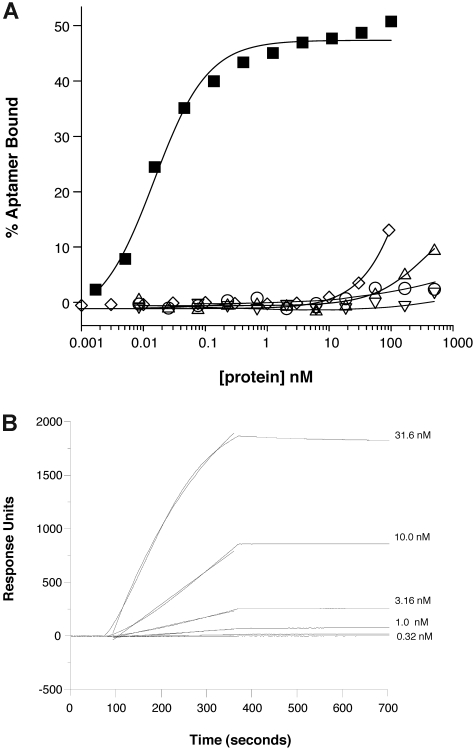
Figure 3.
Anti–P-selectin aptamer binds to murine P-selectin with high specificity. (A) Nitrocellulose filtration assays were performed with 5′-32P-radiolabeled ARC5690 (lacking 5′-PEG) in the presence of increasing concentrations of mouse P-selectin (■), E-selectin (○), L-selectin (▵), or human P-selectin (▿). The aptamer binds to mouse P-selectin with a dissociation constant (KD) of approximately 15pM, and to other proteins with KD > 500nM (the highest protein concentration tested). Radiolabeled scrambled aptamer binds to mouse P-selectin (◇) with KD > 100nM (the highest protein concentration tested). (B) Surface plasmon resonance assay was performed with 5′-biotin–labeled ARC5690 (lacking 5′-PEG) immobilized to a streptavidin-derivatized microfluidics chip. Different concentrations of recombinant murine P-selectin (31.6, 10.0, 3.16, 1.00, and 0.32nM) were injected over the aptamer-coated surface. Surface plasmon resonance data were used to estimate a bimolecular association rate constant (kA) of 2 × 105M−1s−1 and dissociation rate constant (kD) of < 1 × 10−4/s (t1/2 > 2 hours) corresponding to a KD estimate of < 0.5nM.